01 Pages : 1-15
Abstrict
The development of oral insulin presents significant promise for diabetes treatment, mitigating peripheral hyperinsulinemia, weight gain, and hypoglycemia while enhancing patient convenience and facilitating rapid insulinization of the liver. Effective oral insulin products are crucial for early intensive insulin therapy, which ensures tight glycaemic control and delays diabetes complications. However, despite the need, oral insulin has faced many challenges, and past technologies have had limited success. Repeated insulin injections can result in local hypertrophy over time generating attention to user-friendly oral insulin delivery systems that are not invasive to the body and copy the natural pathway. This review article examines New methods for oral insulin delivery that have shown promise, using techniques like complexation, hydrogels, and nanoparticles to boost effectiveness, though they still trail injectable insulin. Further studies will help determine how well oral insulin works at various doses and inform the development of competing formulations.
Keywords
Oral Insulin; Diabetes-Mellitus; Noval Insulin Preparation; Insulin Formulation Technology; Bioavailability
Introduction
Release and Uptake of Insulin through Novel Formulations
The insulin delivery systems that utilize nanocarriers are of huge importance in the field of drug delivery (Xi Z, 2021). These nanocarriers are designed to encapsulate insulin inside their structures. All of them have different drug-release mechanisms. Some of them allow slow insulin diffusion through their walls like the penetration of water from sponges (Taraghdari, 2019). Other mechanisms include rapid release just like the quick flow of liquid from a water-filled balloon when it bursts. These nanocarriers have the capability of accurate spatiotemporal control over drug release. They are designed to identify optimal sites and timing for insulin release, hence ensuring the target delivery process (Karmakar, 2023). As a result, this targeted approach improves the therapeutic efficacy and reduces the possibility of systemic side effects. Post-insulin release then commences a complex intracellular pathway (Guo, 2019). Different endocytic pathways facilitate the uptake of insulin by cells. After entering the cell, insulin enters the labyrinthine network of intracellular compartments.
Figure 1
Mechanism of Action of Oral Insulin
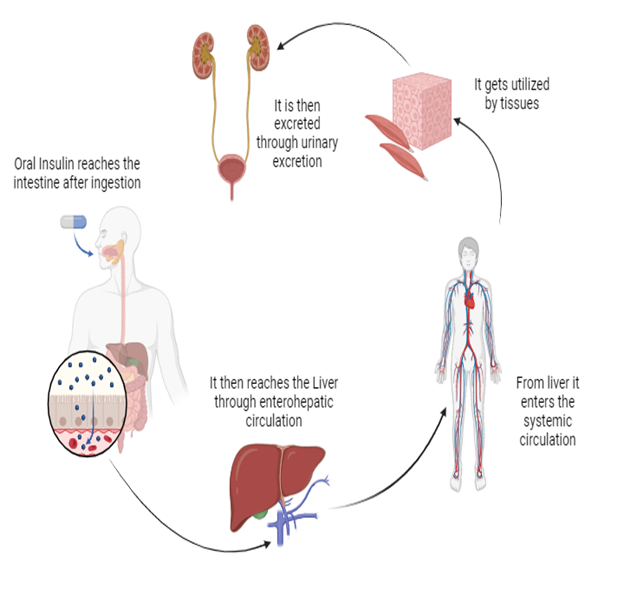
These pathways have endosomes and different vesicular structures that refer to the insulin toward its intracellular targets. The intracellular pathway of insulin consists of its movement through the cytoplasm. In the cytoplasm, it faces different controlled pathways and mechanisms including active transport mechanisms that are mediated via cytoskeletal elements, vesicle-mediated transport, and diffusion from the cytoplasmic matrix (Liu C, 2018). The overall objective of this complex pathway is the targeted delivery within the cell, particularly the intracellular receptors or signaling pathways crucial for its biological activity. The use of nanocarriers in insulin delivery offers substantial promise for improving therapeutic outcomes. Through targeted and controlled release of insulin, these nanocarriers can greatly increase glycemic control in diabetic patients. The accuracy and reliability of these delivery systems represent a remarkable change in diabetes management, particularly in reducing the frequency and severity of hyperglycemic events. (Boushra M, 2019)
Challenges in Oral Insulin Delivery
The oral insulin administration has to confront tremendous challenges, particularly because of the unkind surroundings the gastrointestinal (GI) tract has and the physicochemical properties of the insulin molecule. The route of insulin after reaching the GI tract is followed by a number of factors like deterioration due to enzymes and physical barriers (S. A. S., 2021). All these challenges result in a reduction of insulin bioavailability. Inside the GI tract, insulin is very susceptible to degradation due to enzymes. There are certain enzymes e.g. Pancreatin, aminopeptidases, and pepsin that play a very important part in the enzymatic deterioration of insulin (Pratap-Singh, 2023). Pepsin plays a role in the breakdown of protein in an acidic environment and the degradation process is continued by the enzymes such as chymotrypsin and trypsin accompanied by aminopeptidases that come under the category of pancreatic enzymes. This enzymatic breakdown reduces the bioavailability of insulin drugs that are administered into the oral cavity (JJ, 2022). Insulin has limited permeability for the intestinal epithelium, and it is the other main factor that reduces its bioavailability. The intestinal mucosa has various tight junctions between epithelial cells, that play a role in the restriction of the para-cellular transport of macromolecules such as insulin. Moreover, the passage through the GI tract forces insulin to face different pH environments (Reix, 2012). The acidic condition of the stomach increases the denaturation and degradation of insulin and hence because of its large molecular size and limited lipid solubility, insulin struggles to maintain stability and integrity. The intestinal mucus layer creates an additional challenge to the effective transport of insulin. This viscous barrier has a negative charge and can trap insulin molecules. As a result, it prevents them from reaching the epithelial surface (James, 2014) The defensive function of the mucus layer is significant for the health of the intestines but also helps in the reduction of therapeutic peptides and absorption of protein e.g. insulin. Attainment of increased bioavailability of insulin is still a very critical objective to focus on in the profession of drug delivery (Abdelkadera, 2018). New research concentrates on increasing the bioavailability of insulin from 30% to 50%. There are different creative techniques that are taken into consideration for example, enzyme inhibitors, permeability enhancers, and advanced nanocarrier systems in order to safeguard insulin from deterioration and to assist in its absorption process through the intestinal epithelium (Homayun, 2019).
Innovations in Oral Insulin Delivery
Despite tremendous oral insulin delivery challenges, a variety of innovative techniques are being introduced to enhance the stability and absorption of insulin while increasing its oral bioavailability (Chen, 2018). These revolutions involve using nanoparticle formulations, hydrogels, liposomes, and microspheres (Zhang, 2017). Nanoparticles appreciably shield insulin from enzymatic degradation and facilitate the absorption of the same through the intestinal transitional epithelia. Insulin bioavailability is increased by nanocarriers to a great level, providing more beneficial therapeutic outcomes (Banerjee, 2018). The controlled release mechanism provided by hydrogels safeguards the insulin from the destructive surroundings of the gastrointestinal tract. These systems play an important part in insulin stability and assist its release in a sustained manner. This will overall improve its bioavailability (Mukhopadhyay & Kundu, 2019) Liposomal formulations increase the stability of insulin and help in its transport from the intestinal barrier. Encapsulation of insulin within lipid bilayers protects it from the GI environment and enhances its absorption, thereby improving bioavailability. (Olorunsola, 2021) Microspheres loaded with insulin are designed to prolong its release and improve its bioavailability within the gastrointestinal tract. These systems provide sustained release of insulin and hence optimize its therapeutic efficacy (Strathmann, 2009). Chitosan-based micro- and nanospheres have shown significant promise in improving the mucosal transport of insulin. (Urimi, 2019). The mucoadhesive properties of Chitosan and its biocompatibility make it the most suitable carrier for improving insulin absorption across the mucosal lining (Chellathurai, 2023).
Figure 2
The Physiological Absorption Barriers for the Oral Insulin Administration
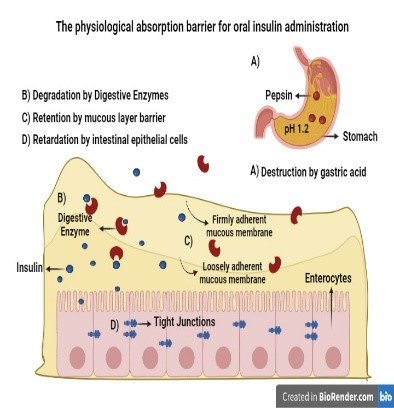
Interactions and Innovations in Polymer-Based Oral Insulin Delivery Systems
The interactions between insulin and polymer substrates exhibit variability in pull-off forces dependent on ambient humidity levels. At 80% relative humidity, consistent adhesion is observed, attributable to surface hydration effects and the mitigation of static charges (Strathmann, 2009). Despite the challenges posed by protein corona formation and nano-protein interactions on the in vivo performance of nanoparticle systems, these phenomena also offer opportunities for the development of advanced drug delivery systems (Zhang, 2020). Chitosan (CS) polymers play a crucial role in stabilizing insulin's structure. Modifications of CS with cholesterol moieties have the potential to alter the affinity between insulin and CS (Seyam, 2020). Insulin-polymer complex formation is largely dependent on critical interactions involving tyrosine, phenylalanine, and acidic residues. These interactions are influenced by a variety of forces, including Van-Der Waals interaction, electrostatic interactions, and CH-? interactions. (Salar, 2018) Nanoparticle self-assembly occurs spontaneously through electrostatic interactions between cationic CS and anionic polymers such as insulin. The given method of encapsulation is classified as non-hexane, which doesn’t pose a problem in terms of non-complexity and comparatively gentle process conditions. (Veloso, 2023). The derived nanoparticles enhance the transport of insulin across the intestinal barrier and protect the loaded insulin from degradation by the enzymes of the gastrointestinal tract. When used in oral insulin delivery systems, these nanoparticles exhibit the ability to improve the insulin's bioavailability and consequently the treatment efficacy in diabetes mellitus (Y., 2015). These advanced delivery devices tackle most of the core issues related to oral insulin delivery by improving the stability and absorption of insulin (Chellathurai, 2023)
Microspheres
Many different modern researchers like Yang, Kim, Zhou, and so forth, have been searching for many creative ways to construct delivery systems for insulin. Their work focuses on using types of microspheres to improve the stability, effectiveness, and availability of insulin when taken orally (Wang, 2018) For instance, the Yangs team created hydrogel microparticles made from chitosan using chemical processes for crosslinking and grafting carboxymethyl ? cyclodextrin. These hydrogels had swelling properties and a porous structure that helped release insulin in the intestines (Mansoor S, 2021). They successfully kept insulin in the system without causing harm. Showed efficient passage through cell layers leading to a significant drop in blood sugar levels in diabetic mice compared to injecting insulin under the skin (Mumuni et al., 2017). In another study Kim et al. Explored chitosan-based insulin formulations linked with acid through interactions. These microspheres had encapsulation rates. Released insulin gradually, after passing through the stomach (Elkordy et al., 2023). Administering these microspheres orally to rats having diabetes, resulted in lasting effects of blood sugar levels over time. The tiny spheres didn't cause harm to cells. Showed the ability to pass through intestinal cell layers hinting at their potential, as a viable option for delivering insulin orally (Yin, 2018). In their study, Zhou and colleagues introduced microspheres that contained metal-organic framework (MOF) nanoparticles, for insulin delivery. The MOF nanoparticles, treated with sodium dodecyl sulfate were able to cross barriers effectively (Malik, 2022). By being enclosed in these spheres' insulin was shielded from conditions. Released gradually over time.
Nanoparticles
Innovative techniques developed by Benyettou (2021) and Chen (2018) for oral insulin delivery, direct the restrictions of conventional administration methods of insulin. Two definite methods evolved from their analysis: insulin-loaded nanoparticles coated with folic acid for virus-mimicking and gastro-retentive nanoparticles using covalent organic frameworks (Xu, 2018). Gastroprotective nanoparticles using an interlinked Covalent Organic Framework(nCOF) evolved by a researcher (Benyettou F, 2021) Insulin was interposed between the sheets of nanoparticles in preference within the penetrable medium of the skeleton. The lucid and passable network of nCOFs revealed a high capacity for insulin wrapping. In vitro studies demonstrated that These nanoparticles effectively conserved insulin in the gastric surroundings and in reaction to low levels of blood glucose released insulin particularly, demonstrating their capacity for targeted and controlled delivery of insulin. (Gelperina S, 2005) Cheng et al. focused on the design of insulin-loaded nanoparticles for oral therapy, utilizing a virus-mimicking approach with folic acid (FA) coating. (Tsai LC, 2018) The core of these nanoparticles consisted of poly (n-butyl cyanoacrylate) loaded with insulin, which was subsequently coated with a negatively charged hyaluronic acid layer and a positively charged Folic Acid - Grafted Chitosan Copolymer (FA-CS). (Jamwal S, 2018) This configuration exhibited insulin release profiles aligned with the physiological conditions of the gastrointestinal tract, providing remarkable stability (Xiao Y, 2021). Nanoparticles with a higher folic-acid graft ratio demonstrated enhanced permeability across the duodenal epithelium. In vivo experiments in diabetic rats revealed superior hypoglycemic effects compared to conventional insulin administration methods, indicating the potential of FA-decorated virus-mimicking nanoparticles to improve diabetes management (Prusty & Sahu, 2013).
Figure 3
Ideal Nanoparticle Properties for the Delivery of Oral Insulin
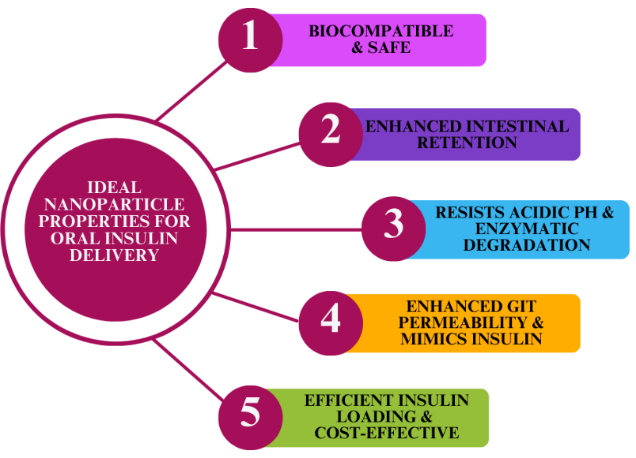
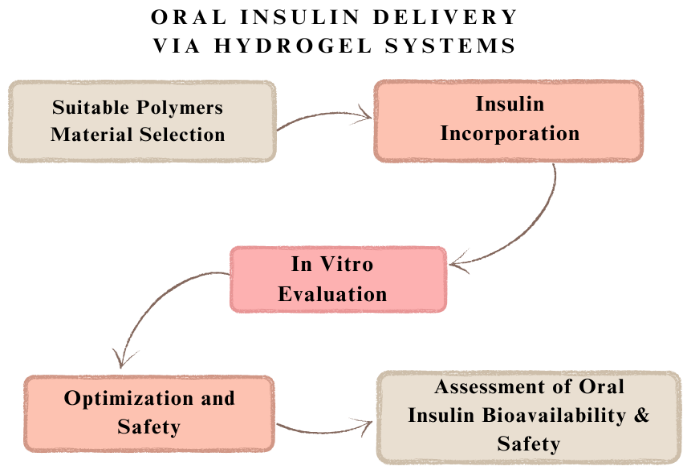
Hydrogels are hydrophilic polymers characterized by three-dimensional viscoelastic networks capable of swelling to many times their dry weight in water under physiological conditions. (Jacob S, 2021) Hydrogels are perfect for biomaterial applications because of their special qualities, which include a high water content, a soft and elastic texture, and a low adhesion force with biological fluids. (Lopes M, 2015) These attributes enable controlled dissolution, protection of labile drugs from degradation, and regulated release of various molecules. Hydrogels typically range in nanoscale dimensions from 10 to 1000 nm. (S. G. P.-S., 2019)
Natural polymers such as chitosan (CS), starch, cellulose, psyllium, and pectin have been utilized to fabricate pH-adaptive hydrogel delivery systems for oral insulin. (Qi X, 2018) Hydrogels' mucoadhesive properties contribute to their safety and efficacy in oral drug delivery, delaying drug absorption and release, and preventing enzymatic degradation of the encapsulated drugs. (Thang NH, 2023) One significant limitation of hydrogel-based insulin delivery devices is their inability to provide prolonged insulin release, often necessitating replenishment after only one or two releases. An effective delivery system should enable at least ten releases before requiring additional administration. Hydrogels also need to compress and expand without hysteresis in order to guarantee steady insulin delivery. This challenge can be mitigated by using softer hydrogel materials and polyprotein cross-linkers. (Li BX, 2021)
Cellulose nanocrystals (CNCs) have been selected for hydrogel fabrication due to their exceptional mechanical qualities, hydrophilicity, biodegradability, low density, and biocompatibility. Acrylic acid monomers were cross-linked in a CNC solution to produce a semi-interpenetrating polymer network, which resulted in a pH-responsive hydrogel. (A., 2010) Chemical cross-linking requires silyl, carboxyl, or aldehyde groups to be added to the surface of CNCs in order to covalently attach them to polymer chains. Direct chemical alteration or physical interaction with CNC surfaces can be used to accomplish this modification. (Goo YT, 2022) Notwithstanding the advantages, unreacted small-molecule cross-linkers can be dangerous, and covalent binding and the first burst release in hydrogel entrapment techniques may deactivate the medication. (George & N, 2015)
In one investigation, a source diffusion technique was used to integrate insulin into the poly (methyl acrylate) (PMA)/salecan/PMA polymer network. In both simulated gastric fluid (SGF) and simulated intestinal fluid (SIF), the pH-dependent release of insulin from these hydrogels was assessed. Following a 24-hour period, the total insulin release for PMA and salecan/PMA hydrogels was 19.7% and 21.5% in SGF and 32.1% and 49.4% in SIF, respectively. When compared to oral free insulin solution, insulin-loaded salecan/PMA hydrogels demonstrated a bioavailability that was more than ten times higher. (Qi X, 2018)
Oral insulin delivery has been tried with cross-linked poly (methacrylic acid) and poly (ethylene glycol) hydrogels (P(MAA-g-EG)). These hydrogels exhibit rapid insulin release in the gut due to their pH-dependent complexing ability, high insulin loading efficiency, mucoadhesive properties, and enzyme inhibition without compromising the intestinal epithelial barrier. P(MAA-g-EG) hydrogel microparticles inhibit gastrointestinal proteolytic enzymes and exhibit intestinal mucoadhesive properties, enhancing oral insulin delivery effectiveness (Fukuoka, 2018)
Cross-linking agents like glutaraldehyde and CaCl2 are employed to create hydrogel structures with alginate and alginate-gelatin matrices. Insulin-loaded alginate beads exhibit a porous structure, which may lead to insulin leakage and low encapsulation efficiency. However, cross-linking alginate-gelatin with glutaraldehyde improves encapsulation efficiency and resistance to gastric conditions. FTIR spectroscopy confirms the stability of insulin within these matrices. MM2 simulations further demonstrate the effects of cross-linking agents and matrix compositions on insulin stability, indicating the potential for optimized drug release in the gastrointestinal system (Gong, 2021)
Sodium carboxymethyl cellulose (CMC), Zingiber officinale polysaccharide (ZOP), and chitosan (CS) have been used to create hydrogel beads for oral insulin delivery. (Olorunsola EO D. K., 2022) These hydrogels exhibit strong pH sensitivity, enabling controlled drug release with high entrapment efficiency (EE) and drug loading rate (DL). In vitro studies show minimal insulin release in SGF and substantial release in SIF, demonstrating their potential as effective oral insulin carriers. (S. G. P.-S., 2019)
A novel pH-regulated hydrogel system utilizing fenugreek seed mucilage extract (FA) was also developed. Insulin was either incorporated as insulin-montmorillonite-Na+ complexes (I-MT) or directly into the hydrogels (I-FA) to create nanocomposite (NC) hydrogels (I-MT-FA). Characterization confirmed the safety and stability of these formulations. (Mohanty AR, 2022) In vivo, evaluations indicated significantly improved relative bioavailability, with I-MT-FA6 NC hydrogel demonstrating a 50.65-fold increase compared to oral insulin solution. Acute oral toxicity studies confirmed the safety and biocompatibility of these hydrogel networks (Wang, 2020).
Figure 4
Oral Insulin Delivery Via Hydrogel System
Ionic Liquids in Pharmaceutical Preparations
The pharmaceutical industry faces significant challenges in addressing the solubility, bioavailability, permeability, polymorphism, and stability of solid-state drugs (A. A.-L. C., 2014) Ionic liquids (ILs) are important participants in handling the problems as therapeutic liquids, reagents, and solvents, anti-solvents in drug formation and crystallization, and as solvents, co-solvents, and stabilizers in drug development (George, 2015). ILs are organic salts made up of cations and anions, causing the outline of compounds with particular biological and physical properties. Regardless of their ability, ILs show side effects such as toxicity, sensitivity to temperature and pH, and challenges with premium burst release rates during storage, notably affecting inert lithium storage, particularly at low temperatures (Almeida, 2023).
ILs, are particularly used in advanced drug delivery methods and are made up of both natural and non-natural compounds. One particular application is the use of a room-temperature stable deep eutectic choline-geranate (CAGE) solvent for insulin delivery. (Berton & Shamshina, 2023)
CAGEs have shown effectiveness in the release of insulin both immediately and over extended periods. In a study, non-diabetic rats were administered 10U/kg of insulin CAGE or its control in enteric-coated capsules of oblong shaped through oral nasogastric tubes. The group given 10U/kg of insulin CAGEs showed a 38% decrease in blood glucose levels within two hours of drug administration. Biocompatible choline-based ILs are showing potential for oral insulin delivery (de Lemos Vasconcelos, 2018) Particularly, choline and geranate (CAGE) ILs have been found to enhance the stability and efficacy of oral insulin. However, the precise molecular interactions between these ILs and insulin remain unclear. To elucidate these interactions, atomistic molecular dynamics (MD) simulations were conducted. (Bala, 2021) The stability of insulin dimers was evaluated in the presence of various CAGE IL concentrations (0.05–1.00 mole fraction) (R., 2022). Experimental observations indicated that CAGE nanostructures in an aqueous medium rearrange at a 0.50 mole fraction. Simulations revealed that choline and geranate ions readily occupy insulin's initial solvation shell in the presence of water. Interestingly, at 0.30–0.50 mole fraction, geranate ions form strong interactions with water molecules, disrupting insulin's intermolecular hydrogen bonds. This optimal concentration range (0.30–0.50 mole fraction) enhances the electrostatic environment around the insulin dimer by promoting water-mediated hydrogen bonding interactions with geranate ions (Shukla, 2023).
Mucoadhesive Patches in Intestinal Drug Delivery
Mucoadhesive patches combining mucoadhesive polymers with water-impermeable backings have been developed to enhance adherence to the intestinal mucosa and improve intestinal permeability. (S, 2002) These patches aim to prolong residence time at the absorption site, thereby increasing the bioavailability, accessibility, and response time of the dosage form. However, despite their potential benefits, mucoadhesive drug delivery systems face several challenges, including the risk of localized ulcers, the lack of suitable in vitro screening models for drug selection, and low patient acceptance due to potential irritation. (Cordery SF, 2019)
Hydrophobic polymers, which resist dissolution in saliva, combined with neutral or positively charged nanoparticles (NPs), demonstrate improved drug efficiency and efficacy in buccal distribution. (Golshani S, 2022) These formulations exhibit stronger adherence to the negatively charged mucosal surface, attributed to sialic acid in the mucus (Banerjee A C. R., 2019). Unidirectional films and tablets, compared to other buccal delivery vehicles and sprays, offer the highest bioavailability. Their ability to mitigate saliva effects and unintended gastrointestinal enzyme digestion minimizes drug loss, contributing to their advantageous characteristics. (Sahni J, 2008)
Intestinal iontophoretic delivery devices, incorporating mucoadhesive insulin patches with integrated circuits and on-chip batteries, offer site-specific drug delivery. These devices can be encapsulated in enteric-coated capsules for controlled release. (Mahajan P, 2013) In a study utilizing Caco-2 cells, electric current-mediated transport of Fluorescein Isothiocyanate (FITC)-insulin demonstrated enhanced uptake, with significantly higher transport observed after two hours compared to the control group. By the end of the five-hour trial, electrically assisted transport facilitated the passage of approximately 14.1 g of FITC-insulin through the cells, compared to 5.5 g in the control group. (Boddupalli et al., 2010)
Encapsulation Strategies for Enhanced Insulin Delivery
Encapsulation within biocompatible nanocarriers offers a promising avenue for facilitating insulin transport across the intestinal mucosa, primarily due to the small particle size enabling paracellular or transcellular transport. Various materials such as nanoparticles (NPs), collagen, poly nanocapsules, and alginate beads have been explored for their potential in insulin delivery and encapsulation. Insulin-loaded NPs typically exhibit a mean diameter of around 180 nm, enhancing their efficacy and reducing adverse effects. (Chen Y, 2018) A novel approach involves encapsulating insulin within metal-organic framework (MOF) crystals. Insulin NU-1000, produced by immersing 2 mg of MOF crystals in an insulin solution, demonstrates significant protection of insulin from the acidic stomach environment, with only 10% release in simulated gastric fluid (SGF) after 60 minutes (Son GH, 2017) However, contact with the simulated intestinal fluid (SIF) triggers rapid insulin release, with 91% released after one hour, suggesting potential for targeted intestinal delivery. Insulin-loaded poly(lactic-co-glycolic acid) (PLGA) nanoparticles (NPs) are produced using a modified double emulsion solvent evaporation technique, with polyvinyl alcohol (PVA) in the outer aqueous phase. The addition of N-ethylmaleimide enhances hypoglycemic effects compared to free insulin, highlighting the efficacy of PLGA NPs as insulin carriers. (C., 2011) CS-NP systems exhibit strong interactions with insulin, particularly due to the hydrophobic nature of cholesterol moieties. These systems demonstrate a preference for peptide hormones and utilize van der Waals, electrostatic, and CH interactions in the encapsulation process, suggesting their potential as superior insulin transporters. ORLN systems effectively encapsulate insulin and protect it from trypsin degradation by forming phospholipid vesicles in the intestinal fluid. Additionally, ORLN-PHI (peptide recombinant human insulin) enhances oral insulin absorption in rats compared to subcutaneously administered free PHI, indicating the potential of ORLN as a nanocarrier for improving insulin oral absorption. (Balasubramanian et al., 2015)
Complexation Strategies for Improving Oral Insulin Bioavailability
Proteins and peptides pose challenges in medical research due to their susceptibility to enzymatic degradation and poor gut absorption, limiting their formulation for oral administration. Current oral protein medications are primarily microbial protein oral vaccinations, with limited applications of liposomes and stabilized foams in medicine. (Munnangi SR, 2023) This study explores the potential of utilizing the biocompatible polymer hyaluronan as a carrier to enhance oral insulin bioavailability. The objective is to optimize an oral insulin formulation process using hyaluronan, leading to the development of a hyaluronan-insulin complex with glucose-lowering properties in diabetic rats. (Sun S, 2008)
All components used in the formulation were sterilized, and native insulin solutions underwent filtration. The formulation process, including vial dispensing, dialysis, pH correction, and mixing, was conducted under aseptic conditions (Chen, 2019). Insulin and hyaluronan were dissolved in an acidic solution and dialyzed in dialysis bags. The physical appearance during complexation was evaluated using light scattering or direct observation, with parameters such as appearance, pH, and ion concentration determined by post-dialysis (Mudassir, 2019). The process involved hyaluronan and insulin dissolution, insulin complexation, and stabilization of the hyaluronan/insulin complex. Hyaluronan required maintenance at a low pH for no longer than 12 hours to prevent molecular disintegration (Ibie CO, 2018). Eleven insulin preparations were developed, each with distinct process parameters, including hyaluronan-to-insulin ratios, duration between additions, dissolution pH, and dialysis medium (Morishita M, 2005). Poly (methacrylic acid) grafted with poly (ethylene glycol) (P(MAA-g-EG)) hydrogels were assessed as potential vehicles for oral insulin delivery. Two insulin-loaded polymers (ILP) sample formulations were investigated, with increasing pH levels leading to higher effective molecular weight between crosslinks () and network mesh size (?) (Rehmani S, 2023). Research on insulin absorption demonstrated high loading efficiency, with insulin release being dose-dependent. In vivo, tests revealed insulin bioactivity, with ILP samples inducing hypoglycemia and elevating insulin levels in rat ileal segments. Insulin absorption within ILP samples varied with polymer quantity and insulin concentration, achieving a maximum relative bioavailability of 8.0% (Mudassir, 2023).
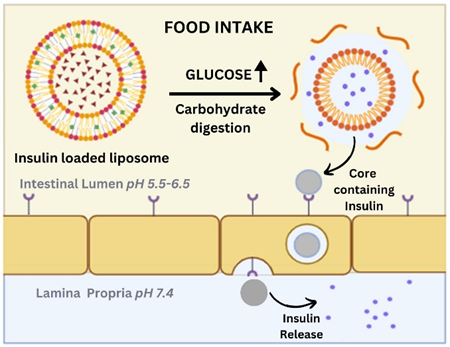
Liposomes
The escalating prevalence of diabetes underscores the urgent need for improved insulin therapy techniques. Current insulin injection methods not only disrupt the body's natural glucose management system but also induce psychological stress. Oral insulin administration holds promise but is hindered by protease degradation, limiting its efficacy (Lee, 2020). Previous research explored various strategies, including detergent micelles, polymer capsules, and oil emulsions, to protect insulin. Recently, attention has shifted to liposomes—biodegradable phospholipid vesicles—as drug-encapsulating carriers. This review highlights the progress and challenges in liposomal insulin formulations, emphasizing the impact of lipid composition, surface charge, and physical properties on their efficacy (Hatakeyama, 2013).
Intragastric delivery of liposomal insulin has demonstrated significant reductions in blood glucose levels. Formulations containing negatively charged or high-melting lipids exhibit enhanced efficacy. Surface-coated liposomes, particularly those coated with polyethylene glycol (PEG) or mucin, exhibit improved stability and resistance to bile salt digestion in vitro. Coated liposomes prevent insulin degradation in intestinal fluid and offer sustained release, leading to prolonged hypoglycemic effects in animal models. Notably, positively charged liposomes showed rapid plasma glucose reduction but lacked sustained hypoglycemic effects (Liu, 2021).
PEGylation and high transition temperature phospholipids, combined with folic acid (FA) targeting, enhance liposomal formulation efficacy. Characterization studies reveal negatively charged liposomes with sizes ranging from 150 to 210 nm. FA conjugation improves insulin uptake without significantly altering transepithelial electrical resistance (TEER). Biodistribution analysis demonstrates prolonged gastric and intestinal residence times for PEGylated liposomes targeted with FA, with increased accumulation in the liver and bloodstream. In vivo studies highlight the anti-diabetic effects of PEGylated liposomes targeted with FA, leading to elevated insulin levels and reduced blood glucose levels. (Wu H, 2022)
Figure 5
Mechanism of Release of Insulin-Loaded Liposomes
Limitations of the Study
The review articles included in this study exhibit limitations pertaining to objectivity, the comprehensiveness of the literature search, and the interpretation of findings. A notable constraint is the absence of a predefined research question or a structured search strategy. Without a clear focus or systematic approach, there is a risk of overlooking relevant research or introducing biases during the selection process. Moreover, the unavailability of any guidelines or criteria for this review may result in variability in data extraction and analysis. Thus, while reviewers may achieve insights into the topic, the review may not offer a detailed understanding of the current landscape of the science of oral insulin delivery.
Future Perspectives
For diabetes mellitus (type 1) management, Insulin therapy
is the gold standard. However, due to the repeated need to inject insulin, the patient's adherence to the therapy is reduced as a result of complications associated with it (Kalaydina, 2018). Despite various non-invasive formulations like oral insulin being devised, none of them are commercially available. Future researchers have to focus on toxicity assessments, monitoring of blood insulin levels, and investigations into dose-dependent therapeutic efficacy for making the oral formulations of insulin easily available for commercial use. (Wong CY, 2017) The innovative Nano delivery system of insulin has promising possibilities for overcoming the current challenges associated with injectable insulin leading to user user-friendly and more convenient approach to insulin therapy. This will also lead to the enhancement of patient compliance as the newer formulations will not be associated with discomfort as injectable insulins are. (El Leithy ES, 2019) Moreover, to overcome existing limitations, upcoming evaluations must create precise research queries, employ systematic search methodologies, adhere to established protocols, and ensure impartial interpretation of findings to provide reliable and informative insights into the subject matter
Conclusion
Alternative non-invasive formulation strategies for insulin therapy have turned up as promising solutions to overcome the limitations associated with the traditional injectable insulin methods and improve patient adherence. The exploration of oral insulin delivery systems has demonstrated significant progress in navigating the challenging gastrointestinal environment, enhancing membrane permeability, and achieving hypoglycaemic effects. To increase the effectiveness and efficiency of oral insulin administration, novel strategies including hydrogels, NPs, microspheres, liposomes, encapsulations, mucoadhesive patches, hydrogels, ionic liquids (ILs), and complexation have been explored. These methods have improved over traditional oral insulin administration, although they are still not as effective as subcutaneous insulin delivery. All these methods are advanced over oral insulin delivery yet they are not as efficient as subcutaneous insulin delivery. Especially those based on NP, especially those utilizing chitosan (CS) outstanding efficiency of NP is attributed to the possibility of an increase in the bioavailability of insulin. In recent years, Nano Particle-based solutions particularly the one, that has incorporated chitosan (CS) have received a lot of appreciation from the scientific community because of their ability to enhance the bioavailability of insulin. However, persistent barriers related to the multifaceted requirements of diabetic persons, rising costs, poor permeability, and biopharmaceutical availability, predicaments of kit stability, and existing ADRs put an imperative on the continued need for an ideal insulin delivery system. It is thus noteworthy that further research activities and advances in this regard may significantly transform the management of the disease and the quality of life of millions of patients in the future.
References
-
Almeida, C., Pedro, A. Q., Tavares, A. P. M., Neves, M. C., & Freire, M. G. (2023). Ionic-liquid-based approaches to improve biopharmaceuticals downstream processing and formulation. Frontiers in Bioengineering and Biotechnology, 11. https://doi.org/10.3389/fbioe.2023.1037436
- Elkordy, A. A., Parveen, A., & Haj-Ahmad, R. (2023). Route of monoclonal antibodies administration. In Elsevier eBooks (pp. 209–258). https://doi.org/10.1016/b978-0-12-823365-8.00005-0
- Taraghdari, Z. B., Imani, R., & Mohabatpour, F. (2019). A review on Bioengineering Approaches to Insulin Delivery: A Pharmaceutical and Engineering perspective. Macromolecular Bioscience, 19(4). https://doi.org/10.1002/mabi.201800458
- Bala, R., Sindhu, R. K., Kaundle, B., Madaan, R., & Cavalu, S. (2021). The prospective of liquid crystals in nano formulations for drug delivery systems. Journal of Molecular Structure, 1245, 131117. https://doi.org/10.1016/j.molstruc.2021.131117
- Banerjee, A., Chen, R., Arafin, S., & Mitragotri, S. (2019). Intestinal iontophoresis from mucoadhesive patches: a strategy for oral delivery. Journal of Controlled Release, 297, 71–78. https://doi.org/10.1016/j.jconrel.2019.01.037
- Banerjee, A., Ibsen, K., Brown, T., Chen, R., Agatemor, C., & Mitragotri, S. (2018). Ionic liquids for oral insulin delivery. Proceedings of the National Academy of Sciences, 115(28), 7296–7301. https://doi.org/10.1073/pnas.1722338115
- Benyettou, F., & K, N. (2021). In vivo oral insulin delivery via covalent organic frameworks. Chemical Science, 12(17), 12345-12356.
- Boushra, M., Tous, S., Fetih, G., Xue, H., & Wong, H. (2019). Development of bi-polymer lipid hybrid nanocarrier (BLN) to improve the entrapment and stability of insulin for efficient oral delivery. Journal of Drug Delivery Science and Technology, 49, 632–641. https://doi.org/10.1016/j.jddst.2019.01.007
- Chellathurai, M. S., Yong, C. L., Sofian, Z. M., Sahudin, S., Hasim, N. B. M., & Mahmood, S. (2023). Self-assembled chitosan-insulin oral nanoparticles — A critical perspective review. International Journal of Biological Macromolecules, 243, 125125. https://doi.org/10.1016/j.ijbiomac.2023.125125
- Chen, C. C., Baikoghli, M. A., & Cheng, R. H. (2018). Tissue targeted nanocapsids for oral insulin delivery via drink. Pharmaceutical Patent Analyst, 7(3), 121–127. https://doi.org/10.4155/ppa-2017-0041
- Chen, X., Ren, Y., Feng, Y., Xu, X., Tan, H., & Li, J. (2019). Cp1-11 peptide/insulin complex loaded pH-responsive nanoparticles with enhanced oral bioactivity. International Journal of Pharmaceutics, 562, 23–30. https://doi.org/10.1016/j.ijpharm.2019.03.020
- Chen, Y., Li, P., Modica, J. A., Drout, R. J., & Farha, O. K. (2018). Acid-Resistant Mesoporous Metal–Organic Framework toward Oral Insulin Delivery: Protein Encapsulation, Protection, and Release. Journal of the American Chemical Society, 140(17), 5678–5681. https://doi.org/10.1021/jacs.8b02089
- Cordery, S. F., Husbands, S. M., Bailey, C. P., Guy, R. H., & Delgado-Charro, M. B. (2019). Simultaneous transdermal delivery of buprenorphine hydrochloride and naltrexone hydrochloride by iontophoresis. Molecular Pharmaceutics, 16(6), 2808–2816. https://doi.org/10.1021/acs.molpharmaceut.9b00337
- De Lemos Vasconcelos Silva, E., De Jesus Oliveira, A. C., Patriota, Y. B. G., Ribeiro, A. J., Veiga, F., Hallwass, F., Silva-Filho, E. C., Da Silva, D. A., De La Roca Soares, M. F., Wanderley, A. G., & Soares-Sobrinho, J. L. (2018). Solvent-free synthesis of acetylated cashew gum for oral delivery system of insulin. Carbohydrate Polymers, 207, 601–608. https://doi.org/10.1016/j.carbpol.2018.11.071
-
Leithy, E. S. E., Abdel-Bar, H. M., & Ali, R. A. (2019). Folate-chitosan nanoparticles triggered insulin cellular uptake and improved in vivo hypoglycemic activity. International Journal of Pharmaceutics, 571, 118708.https://doi.org/10.1016/j.ijpharm.2019.118708
- Fukuoka, Y., Khafagy, E., Goto, T., Kamei, N., Takayama, K., Peppas, N. A., & Takeda-Morishita, M. (2018). Combination Strategy with Complexation Hydrogels and Cell-Penetrating Peptides for Oral Delivery of Insulin. Biological and Pharmaceutical Bulletin, 41(5), 811–814. https://doi.org/10.1248/bpb.b17-00951
- Gelperina, S., Kisich, K., Iseman, M. D., & Heifets, L. (2005). The potential advantages of nanoparticle drug delivery systems in chemotherapy of tuberculosis. American Journal of Respiratory and Critical Care Medicine, 172(12), 1487–1490. https://doi.org/10.1164/rccm.200504-613pp
- Golshani, S., Vatanara, A., & Amin, M. (2022). Recent advances in oral mucoadhesive drug delivery. Journal of Pharmacy & Pharmaceutical Sciences, 25, 201–217. https://doi.org/10.18433/jpps32705
- Gong, Y., Mohd, S., Wu, S., Liu, S., Pei, Y., & Luo, X. (2021). PH-Responsive Cellulose-Based microspheres designed as an effective oral delivery system for insulin. ACS Omega, 6(4), 2734–2741. https://doi.org/10.1021/acsomega.0c04946
- Goo, Y. T., Lee, S., Choi, J. Y., Kim, M. S., Sin, G. H., Hong, S. H., Kim, C. H., Song, S. H., & Choi, Y. W. (2022). Enhanced oral absorption of insulin: hydrophobic ion pairing and a self-microemulsifying drug delivery system using a D-optimal mixture design. Drug Delivery, 29(1), 2831–2845. https://doi.org/10.1080/10717544.2022.2118399
- Guo, F., Ouyang, T., Peng, T., Zhang, X., Xie, B., Yang, X., Liang, D., & Zhong, H. (2019). Enhanced oral absorption of insulin using colon-specific nanoparticles co-modified with amphiphilic chitosan derivatives and cell-penetrating peptides. Biomaterials Science, 7(4), 1493–1506. https://doi.org/10.1039/c8bm01485j
- Hatakeyama, H., Akita, H., & Harashima, H. (2013). The Polyethyleneglycol Dilemma: Advantage and Disadvantage of PEGylation of liposomes for systemic genes and nucleic acids delivery to tumors. Biological and Pharmaceutical Bulletin, 36(6), 892–899. https://doi.org/10.1248/bpb.b13-00059
- Homayun, B., Lin, X., & Choi, H. (2019). Challenges and recent progress in oral drug delivery systems for biopharmaceuticals. Pharmaceutics, 11(3), 129. https://doi.org/10.3390/pharmaceutics11030129
- Ibie, C., Knott, R., & Thompson, C. (2018). Complexation of novel thiomers and insulin to protect against in vitro enzymatic degradation – towards oral insulin delivery. Drug Development and Industrial Pharmacy, 45(1), 67–75. https://doi.org/10.1080/03639045.2018.1517776
- Jacob, S., Nair, A. B., Shah, J., Sreeharsha, N., Gupta, S., & Shinu, P. (2021). Emerging role of hydrogels in drug delivery systems, tissue engineering and wound management. Pharmaceutics, 13(3), 357. https://doi.org/10.3390/pharmaceutics13030357
- James, H. P., John, R., Alex, A., & Anoop, K. (2014). Smart polymers for the controlled delivery of drugs – a concise overview. Acta Pharmaceutica Sinica B, 4(2), 120–127. https://doi.org/10.1016/j.apsb.2014.02.005
- Jamwal, S., Ram, B., Ranote, S., Dharela, R., & Chauhan, G. S. (2018). New glucose oxidase-immobilized stimuli-responsive dextran nanoparticles for insulin delivery. International Journal of Biological Macromolecules, 123, 968–978. https://doi.org/10.1016/j.ijbiomac.2018.11.147
- Malik, M. S. J. K. (2022). Novel Drug Delivery System Microsphere: A Review. SAR Journal of Anatomy and Physiology, 3(2), 9–16. https://doi.org/10.36346/sarjap.2022.v03i02.001
- Berton, P., & Shamshina, J. L. (2023). Ionic Liquids as Tools to Incorporate Pharmaceutical Ingredients into Biopolymer-Based Drug Delivery Systems. Pharmaceuticals, 16(2), 272. https://doi.org/10.3390/ph16020272
- Kalaydina, R. V., & B, K. (2018). Recent advances in “smart” delivery systems for extended drug release in cancer therapy. International Journal of Nanomedicine, 13, 123-134.
- Karmakar, S., Bhowmik, M., Laha, B., & Manna, S. (2023). Recent advancements on novel approaches of insulin delivery. Medicine in Novel Technology and Devices, 19, 100253. https://doi.org/10.1016/j.medntd.2023.100253
- Boddupalli, B., Mohammed, Z., Nath, R., & Banji, D. (2010). Mucoadhesive drug delivery system: An overview. Journal of Advanced Pharmaceutical Technology & Research, 1(4), 381. https://doi.org/10.4103/0110-5558.76436
- Li, Bx., Lv, J., Zhang, X., Zhang, C., Guo, S., Ma, R., Wang, H., & Zhang, Y. (2021). Hypoglycemic effect of insulin-loaded hydrogel-nanogel composite on streptozotocin-induced diabetic rats. PubMed, 76(8), 364–371. https://doi.org/10.1691/ph.2021.1344
- Liu, C., Kou, Y., Zhang, X., Dong, W., Cheng, H., & Mao, S. (2018). Enhanced oral insulin delivery via surface hydrophilic modification of chitosan copolymer based self-assembly polyelectrolyte nanocomplex. International Journal of Pharmaceutics, 554, 36–47. https://doi.org/10.1016/j.ijpharm.2018.10.068
- Liu, G., He, S., Ding, Y., Chen, C., Cai, Q., & Zhou, W. (2021). Multivesicular liposomes for Glucose-Responsive Insulin delivery. Pharmaceutics, 14(1), 21. https://doi.org/10.3390/pharmaceutics14010021
- Lopes, M., Simões, S., Veiga, F., Seiça, R., & Ribeiro, A. (2015). Why most oral insulin formulations do not reach clinical trials. Therapeutic Delivery, 6(8), 973–987. https://doi.org/10.4155/tde.15.47
- Mahajan, P., & K, A. (2013). Mucoadhesive drug delivery system: A review. International Journal of Drug Development & Research, 5(1), 1-10.
- Balasubramanian, S., Sampath, M., Perumal, N., Pandiyan, V., & Webster, T. J. (2015). Novel PLGA-based nanoparticles for the oral delivery of insulin. International Journal of Nanomedicine, 2207. https://doi.org/10.2147/ijn.s67947
- Mansoor, S., Kondiah, P. P. D., & Choonara, Y. E. (2021). Advanced hydrogels for the controlled delivery of insulin. Pharmaceutics, 13(12), 2113. https://doi.org/10.3390/pharmaceutics13122113
- Lee, M. (2020). Liposomes for Enhanced Bioavailability of Water-Insoluble Drugs: In Vivo evidence and Recent Approaches. Pharmaceutics, 12(3), 264. https://doi.org/10.3390/pharmaceutics12030264
- Mohanty, A. R., Ravikumar, A., & Peppas, N. A. (2022). Recent advances in glucose-responsive insulin delivery systems: novel hydrogels and future applications. Regenerative Biomaterials, 9. https://doi.org/10.1093/rb/rbac056
- Mumuni, A. M., Tenderwealth, C. J., Adedokun, O. M., Kenechukwu, F. C., Youngson, C. D., & Kenneth, C. O. (2017). Microspheres of insulin-Eudragit complex: Formulation, characterization and in vivo studies. African Journal of Pharmacy and Pharmacology, 11(29), 327–341. https://doi.org/10.5897/ajpp2017.4796
- Morishita, M., Goto, T., Nakamura, K., Lowman, A. M., Takayama, K., & Peppas, N. A. (2005). Novel oral insulin delivery systems based on complexation polymer hydrogels: Single and multiple administration studies in type 1 and 2 diabetic rats. Journal of Controlled Release, 110(3), 587–594. https://doi.org/10.1016/j.jconrel.2005.10.029
- Mudassir, J., Darwis, Y., Muhamad, S., & Khan, A. A. (2019). <p>Self-assembled insulin and nanogels polyelectrolyte complex (Ins/NGs-PEC) for oral insulin delivery: characterization, lyophilization and in-vivo evaluation</p> International Journal of Nanomedicine, Volume 14, 4895–4909. https://doi.org/10.2147/ijn.s199507
- Mudassir, J., Raza, A., Khan, M. A., Hameed, H., Shazly, G. A., Irfan, A., Rana, S. J., Abbas, K., Arshad, M. S., Muhammad, S., & Jardan, Y. a. B. (2023). Design and evaluation of hydrophobic ion paired insulin loaded Self Micro-Emulsifying drug delivery System for oral delivery. Pharmaceutics, 15(7), 1973. https://doi.org/10.3390/pharmaceutics15071973
- Mukhopadhyay, P., & Kundu, P. P. (2019). Stimuli-responsive polymers for oral insulin delivery. In A. S. H. Makhlouf & N. Y. Abu-Thabit (Eds.), Stimuli responsive polymeric nanocarriers for drug delivery applications (pp. 525-546). Springer.
- Munnangi, S. R., Youssef, A. a. A., Narala, N., Lakkala, P., Narala, S., Vemula, S. K., & Repka, M. (2023). Drug complexes: Perspective from Academic Research and Pharmaceutical Market. Pharmaceutical Research, 40(6), 1519–1540. https://doi.org/10.1007/s11095-023-03517-w
- Olorunsola, E. O., & A, M. (2021). Advances in the science and technology of insulin delivery: A review. Journal of Applied Pharmaceutical Science, 11(5), 184–191.
- Olorunsola, E. O., Davies, K. G., Ibiang, K. P., Esukpsa, P. C., Uwaechi, E. G., & Ahsan, F. (2022). Prosochit®-based nanoparticulate system of insulin for oral delivery: design, formulation, and characterization. Journal of Applied Pharmaceutical Science. https://doi.org/10.7324/japs.2023.90862
- Pratap-Singh, A., Guo, Y., Baldelli, A., & Singh, A. (2023). Concept for a Unidirectional Release Mucoadhesive Buccal Tablet for Oral Delivery of Antidiabetic Peptide Drugs Such as Insulin, Glucagon-like Peptide 1 (GLP-1), and their Analogs. Pharmaceutics, 15(9), 2265. https://doi.org/10.3390/pharmaceutics15092265
- Qi, X., Yuan, Y., Zhang, J., Bulte, J. W. M., & Dong, W. (2018). Oral administration of Salecan-Based hydrogels for controlled insulin delivery. Journal of Agricultural and Food Chemistry, 66(40), 10479–10489. https://doi.org/10.1021/acs.jafc.8b02879
- Rehmani, S., McLaughlin, C. M., Eltaher, H. M., Moffett, R. C., Flatt, P. R., & Dixon, J. E. (2023). Orally-delivered insulin-peptide nanocomplexes enhance transcytosis from cellular depots and improve diabetic blood glucose control. Journal of Controlled Release, 360, 93–109. https://doi.org/10.1016/j.jconrel.2023.06.006
- Reix, N., Parat, A., Seyfritz, E., Van Der Werf, R., Epure, V., Ebel, N., Danicher, L., Marchioni, E., Jeandidier, N., Pinget, M., Frère, Y., & Sigrist, S. (2012). In vitro uptake evaluation in Caco-2 cells and in vivo results in diabetic rats of insulin-loaded PLGA nanoparticles. International Journal of Pharmaceutics, 437(1–2), 213–220. https://doi.org/10.1016/j.ijpharm.2012.08.024
- Sahni, J., & R, S. (2008). Design and in vitro characterization of bucco-adhesive drug delivery system of insulin. Indian Journal of Pharmaceutical Sciences, 70(4), 512-516. https://doi.org/10.4103/0250-474X.40333
- Salar, S., Jafari, M., Kaboli, S. F., & Mehrnejad, F. (2018). The role of intermolecular interactions on the encapsulation of human insulin into the chitosan and cholesterol-grafted chitosan polymers. Carbohydrate Polymers, 208, 345–355. https://doi.org/10.1016/j.carbpol.2018.12.083
- Seyam, S., Nordin, N. A., & Alfatama, M. (2020). Recent progress of Chitosan and Chitosan Derivatives-Based Nanoparticles: Pharmaceutical Perspectives of oral insulin delivery. Pharmaceuticals, 13(10), 307. https://doi.org/10.3390/ph13100307
-
Shukla, M. K., Tiwari, H., Verma, R., Dong, W., Azizov, S., Kumar, B., Pandey, S., & Kumar, D. (2023). Role and recent advancements of ionic liquids in drug delivery systems. Pharmaceutics, 15(2), 702. https://doi.org/10.3390/pharmaceutics15020702
- Prusty, A. K., & Sahu, S. K. (2013). Development and evaluation of insulin incorporated nanoparticles for oral administration. ISRN Nanotechnology, 2013, 1–6. https://doi.org/10.1155/2013/591751
- George, J., & N, S. S. (2015). Cellulose nanocrystals: synthesis, functional properties, and applications. Nanotechnology Science and Applications, 45. https://doi.org/10.2147/nsa.s64386
- Son, G., Lee, B., & Cho, C. (2017). Mechanisms of drug release from advanced drug formulations such as polymeric-based drug-delivery systems and lipid nanoparticles. Journal of Pharmaceutical Investigation, 47(4), 287–296. https://doi.org/10.1007/s40005-017-0320-1
- Strathmann, S. C., Murphy, M. A., Goeckner, B. A., Carter, P. W., & Green, J. D. (2009). Forces between insulin microspheres and polymers surfaces for a dry powder inhaler. International Journal of Pharmaceutics, 372(1–2), 147–153. https://doi.org/10.1016/j.ijpharm.2009.01.004
- Thang, N. H., Chien, T. B., & Cuong, D. X. (2023). Polymer-Based Hydrogels Applied in Drug Delivery: An Overview. Gels, 9(7), 523. https://doi.org/10.3390/gels9070523
- Tsai, L., Chen, C., Lin, C., Ho, Y., & Mi, F. (2018). Development of mutlifunctional nanoparticles self-assembled from trimethyl chitosan and fucoidan for enhanced oral delivery of insulin. International Journal of Biological Macromolecules, 126, 141–150. https://doi.org/10.1016/j.ijbiomac.2018.12.182
- Urimi, D., Agrawal, A. K., Kushwah, V., & Jain, S. (2019). Polyglutamic acid functionalization of chitosan nanoparticles enhances the therapeutic efficacy of insulin following oral administration. AAPS PharmSciTech, 20(3). https://doi.org/10.1208/s12249-019-1330-2
- Veloso, S. R. S., Azevedo, A. G., Teixeira, P. F., & Fernandes, C. B. P. (2023). Cellulose nanocrystal (CNC) gels: a review. Gels, 9(7), 574. https://doi.org/10.3390/gels9070574
- Wang, A., Yang, T., Fan, W., Yang, Y., Zhu, Q., Guo, S., Zhu, C., Yuan, Y., Zhang, T., & Gan, Y. (2018). Protein corona liposomes achieve efficient oral insulin delivery by overcoming mucus and epithelial barriers. Advanced Healthcare Materials, 8(12). https://doi.org/10.1002/adhm.201801123
- Wang, T., & S, L. (2020). “Oil-soluble” reversed lipid nanoparticles for oral insulin delivery. Journal of Nanobiotechnology, 18, Article 98.
- Wong, C. Y., Al-Salami, H., & Dass, C. R. (2017). Microparticles, microcapsules and microspheres: A review of recent developments and prospects for oral delivery of insulin. International Journal of Pharmaceutics, 537(1–2), 223–244. https://doi.org/10.1016/j.ijpharm.2017.12.036
- Wu, H., Nan, J., Yang, L., Park, H. J., & Li, J. (2022). Insulin-loaded liposomes packaged in alginate hydrogels promote the oral bioavailability of insulin. Journal of Controlled Release, 353, 51–62. https://doi.org/10.1016/j.jconrel.2022.11.032
- Xi, Z., Ahmad, E., Zhang, W., Li, J., Wang, A., Faridoon, Wang, N., Zhu, C., Huang, W., Xu, L., Yu, M., & Gan, Y. (2021). Dual-modified nanoparticles overcome sequential absorption barriers for oral insulin delivery. Journal of Controlled Release, 342, 1–13. https://doi.org/10.1016/j.jconrel.2021.11.045
- Xiao, Y., Tang, Z., Huang, X., Joseph, J., Chen, W., Liu, C., Zhou, J., Kong, N., Joshi, N., Du, J., & Tao, W. (2021). Glucose-responsive oral insulin delivery platform for one treatment a day in diabetes. Matter, 4(10), 3269–3285. https://doi.org/10.1016/j.matt.2021.08.011
- Xu, Y., Zheng, Y., Wu, L., Zhu, X., Zhang, Z., & Huang, Y. (2018). Novel Solid Lipid Nanoparticle with Endosomal Escape Function for Oral Delivery of Insulin. ACS Applied Materials & Interfaces, 10(11), 9315–9324. https://doi.org/10.1021/acsami.8b00507
-
Yin, R., He, J., Bai, M., Huang, C., Wang, K., Zhang, H., Yang, S., & Zhang, W. (2018). Engineering synthetic artificial pancreas using chitosan hydrogels integrated with glucose-responsive microspheres for insulin delivery. Materials Science and Engineering C, 96, 374–382. https://doi.org/10.1016/j.msec.2018.11.032Google Scholar Worldcat Fulltext
- Zhang, H., Wang, W., Li, H., Peng, Y., & Zhang, Z. (2017). Microspheres for the oral delivery of insulin: preparation, evaluation and hypoglycaemic effect in streptozotocin-induced diabetic rats. Drug Development and Industrial Pharmacy, 44(1), 109–115. https://doi.org/10.1080/03639045.2017.1386197
- Zhang, T., Tang, J. Z., Fei, X., Li, Y., Song, Y., Qian, Z., & Peng, Q. (2020). Can nanoparticles and nano‒protein interactions bring a bright future for insulin delivery? Acta Pharmaceutica Sinica B, 11(3), 651–667. https://doi.org/10.1016/j.apsb.2020.08.016
- Zou, J., Wei, G., Xiong, C., Yu, Y., Li, S., Hu, L., Ma, S., & Tian, J. (2022). Efficient oral insulin delivery enabled by transferrin-coated acid-resistant metal-organic framework nanoparticles. Science Advances, 8(8). https://doi.org/10.1126/sciadv.abm4677
-
Almeida, C., Pedro, A. Q., Tavares, A. P. M., Neves, M. C., & Freire, M. G. (2023). Ionic-liquid-based approaches to improve biopharmaceuticals downstream processing and formulation. Frontiers in Bioengineering and Biotechnology, 11. https://doi.org/10.3389/fbioe.2023.1037436
- Elkordy, A. A., Parveen, A., & Haj-Ahmad, R. (2023). Route of monoclonal antibodies administration. In Elsevier eBooks (pp. 209–258). https://doi.org/10.1016/b978-0-12-823365-8.00005-0
- Taraghdari, Z. B., Imani, R., & Mohabatpour, F. (2019). A review on Bioengineering Approaches to Insulin Delivery: A Pharmaceutical and Engineering perspective. Macromolecular Bioscience, 19(4). https://doi.org/10.1002/mabi.201800458
- Bala, R., Sindhu, R. K., Kaundle, B., Madaan, R., & Cavalu, S. (2021). The prospective of liquid crystals in nano formulations for drug delivery systems. Journal of Molecular Structure, 1245, 131117. https://doi.org/10.1016/j.molstruc.2021.131117
- Banerjee, A., Chen, R., Arafin, S., & Mitragotri, S. (2019). Intestinal iontophoresis from mucoadhesive patches: a strategy for oral delivery. Journal of Controlled Release, 297, 71–78. https://doi.org/10.1016/j.jconrel.2019.01.037
- Banerjee, A., Ibsen, K., Brown, T., Chen, R., Agatemor, C., & Mitragotri, S. (2018). Ionic liquids for oral insulin delivery. Proceedings of the National Academy of Sciences, 115(28), 7296–7301. https://doi.org/10.1073/pnas.1722338115
- Benyettou, F., & K, N. (2021). In vivo oral insulin delivery via covalent organic frameworks. Chemical Science, 12(17), 12345-12356.
- Boushra, M., Tous, S., Fetih, G., Xue, H., & Wong, H. (2019). Development of bi-polymer lipid hybrid nanocarrier (BLN) to improve the entrapment and stability of insulin for efficient oral delivery. Journal of Drug Delivery Science and Technology, 49, 632–641. https://doi.org/10.1016/j.jddst.2019.01.007
- Chellathurai, M. S., Yong, C. L., Sofian, Z. M., Sahudin, S., Hasim, N. B. M., & Mahmood, S. (2023). Self-assembled chitosan-insulin oral nanoparticles — A critical perspective review. International Journal of Biological Macromolecules, 243, 125125. https://doi.org/10.1016/j.ijbiomac.2023.125125
- Chen, C. C., Baikoghli, M. A., & Cheng, R. H. (2018). Tissue targeted nanocapsids for oral insulin delivery via drink. Pharmaceutical Patent Analyst, 7(3), 121–127. https://doi.org/10.4155/ppa-2017-0041
- Chen, X., Ren, Y., Feng, Y., Xu, X., Tan, H., & Li, J. (2019). Cp1-11 peptide/insulin complex loaded pH-responsive nanoparticles with enhanced oral bioactivity. International Journal of Pharmaceutics, 562, 23–30. https://doi.org/10.1016/j.ijpharm.2019.03.020
- Chen, Y., Li, P., Modica, J. A., Drout, R. J., & Farha, O. K. (2018). Acid-Resistant Mesoporous Metal–Organic Framework toward Oral Insulin Delivery: Protein Encapsulation, Protection, and Release. Journal of the American Chemical Society, 140(17), 5678–5681. https://doi.org/10.1021/jacs.8b02089
- Cordery, S. F., Husbands, S. M., Bailey, C. P., Guy, R. H., & Delgado-Charro, M. B. (2019). Simultaneous transdermal delivery of buprenorphine hydrochloride and naltrexone hydrochloride by iontophoresis. Molecular Pharmaceutics, 16(6), 2808–2816. https://doi.org/10.1021/acs.molpharmaceut.9b00337
- De Lemos Vasconcelos Silva, E., De Jesus Oliveira, A. C., Patriota, Y. B. G., Ribeiro, A. J., Veiga, F., Hallwass, F., Silva-Filho, E. C., Da Silva, D. A., De La Roca Soares, M. F., Wanderley, A. G., & Soares-Sobrinho, J. L. (2018). Solvent-free synthesis of acetylated cashew gum for oral delivery system of insulin. Carbohydrate Polymers, 207, 601–608. https://doi.org/10.1016/j.carbpol.2018.11.071
-
Leithy, E. S. E., Abdel-Bar, H. M., & Ali, R. A. (2019). Folate-chitosan nanoparticles triggered insulin cellular uptake and improved in vivo hypoglycemic activity. International Journal of Pharmaceutics, 571, 118708.https://doi.org/10.1016/j.ijpharm.2019.118708
- Fukuoka, Y., Khafagy, E., Goto, T., Kamei, N., Takayama, K., Peppas, N. A., & Takeda-Morishita, M. (2018). Combination Strategy with Complexation Hydrogels and Cell-Penetrating Peptides for Oral Delivery of Insulin. Biological and Pharmaceutical Bulletin, 41(5), 811–814. https://doi.org/10.1248/bpb.b17-00951
- Gelperina, S., Kisich, K., Iseman, M. D., & Heifets, L. (2005). The potential advantages of nanoparticle drug delivery systems in chemotherapy of tuberculosis. American Journal of Respiratory and Critical Care Medicine, 172(12), 1487–1490. https://doi.org/10.1164/rccm.200504-613pp
- Golshani, S., Vatanara, A., & Amin, M. (2022). Recent advances in oral mucoadhesive drug delivery. Journal of Pharmacy & Pharmaceutical Sciences, 25, 201–217. https://doi.org/10.18433/jpps32705
- Gong, Y., Mohd, S., Wu, S., Liu, S., Pei, Y., & Luo, X. (2021). PH-Responsive Cellulose-Based microspheres designed as an effective oral delivery system for insulin. ACS Omega, 6(4), 2734–2741. https://doi.org/10.1021/acsomega.0c04946
- Goo, Y. T., Lee, S., Choi, J. Y., Kim, M. S., Sin, G. H., Hong, S. H., Kim, C. H., Song, S. H., & Choi, Y. W. (2022). Enhanced oral absorption of insulin: hydrophobic ion pairing and a self-microemulsifying drug delivery system using a D-optimal mixture design. Drug Delivery, 29(1), 2831–2845. https://doi.org/10.1080/10717544.2022.2118399
- Guo, F., Ouyang, T., Peng, T., Zhang, X., Xie, B., Yang, X., Liang, D., & Zhong, H. (2019). Enhanced oral absorption of insulin using colon-specific nanoparticles co-modified with amphiphilic chitosan derivatives and cell-penetrating peptides. Biomaterials Science, 7(4), 1493–1506. https://doi.org/10.1039/c8bm01485j
- Hatakeyama, H., Akita, H., & Harashima, H. (2013). The Polyethyleneglycol Dilemma: Advantage and Disadvantage of PEGylation of liposomes for systemic genes and nucleic acids delivery to tumors. Biological and Pharmaceutical Bulletin, 36(6), 892–899. https://doi.org/10.1248/bpb.b13-00059
- Homayun, B., Lin, X., & Choi, H. (2019). Challenges and recent progress in oral drug delivery systems for biopharmaceuticals. Pharmaceutics, 11(3), 129. https://doi.org/10.3390/pharmaceutics11030129
- Ibie, C., Knott, R., & Thompson, C. (2018). Complexation of novel thiomers and insulin to protect against in vitro enzymatic degradation – towards oral insulin delivery. Drug Development and Industrial Pharmacy, 45(1), 67–75. https://doi.org/10.1080/03639045.2018.1517776
- Jacob, S., Nair, A. B., Shah, J., Sreeharsha, N., Gupta, S., & Shinu, P. (2021). Emerging role of hydrogels in drug delivery systems, tissue engineering and wound management. Pharmaceutics, 13(3), 357. https://doi.org/10.3390/pharmaceutics13030357
- James, H. P., John, R., Alex, A., & Anoop, K. (2014). Smart polymers for the controlled delivery of drugs – a concise overview. Acta Pharmaceutica Sinica B, 4(2), 120–127. https://doi.org/10.1016/j.apsb.2014.02.005
- Jamwal, S., Ram, B., Ranote, S., Dharela, R., & Chauhan, G. S. (2018). New glucose oxidase-immobilized stimuli-responsive dextran nanoparticles for insulin delivery. International Journal of Biological Macromolecules, 123, 968–978. https://doi.org/10.1016/j.ijbiomac.2018.11.147
- Malik, M. S. J. K. (2022). Novel Drug Delivery System Microsphere: A Review. SAR Journal of Anatomy and Physiology, 3(2), 9–16. https://doi.org/10.36346/sarjap.2022.v03i02.001
- Berton, P., & Shamshina, J. L. (2023). Ionic Liquids as Tools to Incorporate Pharmaceutical Ingredients into Biopolymer-Based Drug Delivery Systems. Pharmaceuticals, 16(2), 272. https://doi.org/10.3390/ph16020272
- Kalaydina, R. V., & B, K. (2018). Recent advances in “smart” delivery systems for extended drug release in cancer therapy. International Journal of Nanomedicine, 13, 123-134.
- Karmakar, S., Bhowmik, M., Laha, B., & Manna, S. (2023). Recent advancements on novel approaches of insulin delivery. Medicine in Novel Technology and Devices, 19, 100253. https://doi.org/10.1016/j.medntd.2023.100253
- Boddupalli, B., Mohammed, Z., Nath, R., & Banji, D. (2010). Mucoadhesive drug delivery system: An overview. Journal of Advanced Pharmaceutical Technology & Research, 1(4), 381. https://doi.org/10.4103/0110-5558.76436
- Li, Bx., Lv, J., Zhang, X., Zhang, C., Guo, S., Ma, R., Wang, H., & Zhang, Y. (2021). Hypoglycemic effect of insulin-loaded hydrogel-nanogel composite on streptozotocin-induced diabetic rats. PubMed, 76(8), 364–371. https://doi.org/10.1691/ph.2021.1344
- Liu, C., Kou, Y., Zhang, X., Dong, W., Cheng, H., & Mao, S. (2018). Enhanced oral insulin delivery via surface hydrophilic modification of chitosan copolymer based self-assembly polyelectrolyte nanocomplex. International Journal of Pharmaceutics, 554, 36–47. https://doi.org/10.1016/j.ijpharm.2018.10.068
- Liu, G., He, S., Ding, Y., Chen, C., Cai, Q., & Zhou, W. (2021). Multivesicular liposomes for Glucose-Responsive Insulin delivery. Pharmaceutics, 14(1), 21. https://doi.org/10.3390/pharmaceutics14010021
- Lopes, M., Simões, S., Veiga, F., Seiça, R., & Ribeiro, A. (2015). Why most oral insulin formulations do not reach clinical trials. Therapeutic Delivery, 6(8), 973–987. https://doi.org/10.4155/tde.15.47
- Mahajan, P., & K, A. (2013). Mucoadhesive drug delivery system: A review. International Journal of Drug Development & Research, 5(1), 1-10.
- Balasubramanian, S., Sampath, M., Perumal, N., Pandiyan, V., & Webster, T. J. (2015). Novel PLGA-based nanoparticles for the oral delivery of insulin. International Journal of Nanomedicine, 2207. https://doi.org/10.2147/ijn.s67947
- Mansoor, S., Kondiah, P. P. D., & Choonara, Y. E. (2021). Advanced hydrogels for the controlled delivery of insulin. Pharmaceutics, 13(12), 2113. https://doi.org/10.3390/pharmaceutics13122113
- Lee, M. (2020). Liposomes for Enhanced Bioavailability of Water-Insoluble Drugs: In Vivo evidence and Recent Approaches. Pharmaceutics, 12(3), 264. https://doi.org/10.3390/pharmaceutics12030264
- Mohanty, A. R., Ravikumar, A., & Peppas, N. A. (2022). Recent advances in glucose-responsive insulin delivery systems: novel hydrogels and future applications. Regenerative Biomaterials, 9. https://doi.org/10.1093/rb/rbac056
- Mumuni, A. M., Tenderwealth, C. J., Adedokun, O. M., Kenechukwu, F. C., Youngson, C. D., & Kenneth, C. O. (2017). Microspheres of insulin-Eudragit complex: Formulation, characterization and in vivo studies. African Journal of Pharmacy and Pharmacology, 11(29), 327–341. https://doi.org/10.5897/ajpp2017.4796
- Morishita, M., Goto, T., Nakamura, K., Lowman, A. M., Takayama, K., & Peppas, N. A. (2005). Novel oral insulin delivery systems based on complexation polymer hydrogels: Single and multiple administration studies in type 1 and 2 diabetic rats. Journal of Controlled Release, 110(3), 587–594. https://doi.org/10.1016/j.jconrel.2005.10.029
- Mudassir, J., Darwis, Y., Muhamad, S., & Khan, A. A. (2019). <p>Self-assembled insulin and nanogels polyelectrolyte complex (Ins/NGs-PEC) for oral insulin delivery: characterization, lyophilization and in-vivo evaluation</p> International Journal of Nanomedicine, Volume 14, 4895–4909. https://doi.org/10.2147/ijn.s199507
- Mudassir, J., Raza, A., Khan, M. A., Hameed, H., Shazly, G. A., Irfan, A., Rana, S. J., Abbas, K., Arshad, M. S., Muhammad, S., & Jardan, Y. a. B. (2023). Design and evaluation of hydrophobic ion paired insulin loaded Self Micro-Emulsifying drug delivery System for oral delivery. Pharmaceutics, 15(7), 1973. https://doi.org/10.3390/pharmaceutics15071973
- Mukhopadhyay, P., & Kundu, P. P. (2019). Stimuli-responsive polymers for oral insulin delivery. In A. S. H. Makhlouf & N. Y. Abu-Thabit (Eds.), Stimuli responsive polymeric nanocarriers for drug delivery applications (pp. 525-546). Springer.
- Munnangi, S. R., Youssef, A. a. A., Narala, N., Lakkala, P., Narala, S., Vemula, S. K., & Repka, M. (2023). Drug complexes: Perspective from Academic Research and Pharmaceutical Market. Pharmaceutical Research, 40(6), 1519–1540. https://doi.org/10.1007/s11095-023-03517-w
- Olorunsola, E. O., & A, M. (2021). Advances in the science and technology of insulin delivery: A review. Journal of Applied Pharmaceutical Science, 11(5), 184–191.
- Olorunsola, E. O., Davies, K. G., Ibiang, K. P., Esukpsa, P. C., Uwaechi, E. G., & Ahsan, F. (2022). Prosochit®-based nanoparticulate system of insulin for oral delivery: design, formulation, and characterization. Journal of Applied Pharmaceutical Science. https://doi.org/10.7324/japs.2023.90862
- Pratap-Singh, A., Guo, Y., Baldelli, A., & Singh, A. (2023). Concept for a Unidirectional Release Mucoadhesive Buccal Tablet for Oral Delivery of Antidiabetic Peptide Drugs Such as Insulin, Glucagon-like Peptide 1 (GLP-1), and their Analogs. Pharmaceutics, 15(9), 2265. https://doi.org/10.3390/pharmaceutics15092265
- Qi, X., Yuan, Y., Zhang, J., Bulte, J. W. M., & Dong, W. (2018). Oral administration of Salecan-Based hydrogels for controlled insulin delivery. Journal of Agricultural and Food Chemistry, 66(40), 10479–10489. https://doi.org/10.1021/acs.jafc.8b02879
- Rehmani, S., McLaughlin, C. M., Eltaher, H. M., Moffett, R. C., Flatt, P. R., & Dixon, J. E. (2023). Orally-delivered insulin-peptide nanocomplexes enhance transcytosis from cellular depots and improve diabetic blood glucose control. Journal of Controlled Release, 360, 93–109. https://doi.org/10.1016/j.jconrel.2023.06.006
- Reix, N., Parat, A., Seyfritz, E., Van Der Werf, R., Epure, V., Ebel, N., Danicher, L., Marchioni, E., Jeandidier, N., Pinget, M., Frère, Y., & Sigrist, S. (2012). In vitro uptake evaluation in Caco-2 cells and in vivo results in diabetic rats of insulin-loaded PLGA nanoparticles. International Journal of Pharmaceutics, 437(1–2), 213–220. https://doi.org/10.1016/j.ijpharm.2012.08.024
- Sahni, J., & R, S. (2008). Design and in vitro characterization of bucco-adhesive drug delivery system of insulin. Indian Journal of Pharmaceutical Sciences, 70(4), 512-516. https://doi.org/10.4103/0250-474X.40333
- Salar, S., Jafari, M., Kaboli, S. F., & Mehrnejad, F. (2018). The role of intermolecular interactions on the encapsulation of human insulin into the chitosan and cholesterol-grafted chitosan polymers. Carbohydrate Polymers, 208, 345–355. https://doi.org/10.1016/j.carbpol.2018.12.083
- Seyam, S., Nordin, N. A., & Alfatama, M. (2020). Recent progress of Chitosan and Chitosan Derivatives-Based Nanoparticles: Pharmaceutical Perspectives of oral insulin delivery. Pharmaceuticals, 13(10), 307. https://doi.org/10.3390/ph13100307
-
Shukla, M. K., Tiwari, H., Verma, R., Dong, W., Azizov, S., Kumar, B., Pandey, S., & Kumar, D. (2023). Role and recent advancements of ionic liquids in drug delivery systems. Pharmaceutics, 15(2), 702. https://doi.org/10.3390/pharmaceutics15020702
- Prusty, A. K., & Sahu, S. K. (2013). Development and evaluation of insulin incorporated nanoparticles for oral administration. ISRN Nanotechnology, 2013, 1–6. https://doi.org/10.1155/2013/591751
- George, J., & N, S. S. (2015). Cellulose nanocrystals: synthesis, functional properties, and applications. Nanotechnology Science and Applications, 45. https://doi.org/10.2147/nsa.s64386
- Son, G., Lee, B., & Cho, C. (2017). Mechanisms of drug release from advanced drug formulations such as polymeric-based drug-delivery systems and lipid nanoparticles. Journal of Pharmaceutical Investigation, 47(4), 287–296. https://doi.org/10.1007/s40005-017-0320-1
- Strathmann, S. C., Murphy, M. A., Goeckner, B. A., Carter, P. W., & Green, J. D. (2009). Forces between insulin microspheres and polymers surfaces for a dry powder inhaler. International Journal of Pharmaceutics, 372(1–2), 147–153. https://doi.org/10.1016/j.ijpharm.2009.01.004
- Thang, N. H., Chien, T. B., & Cuong, D. X. (2023). Polymer-Based Hydrogels Applied in Drug Delivery: An Overview. Gels, 9(7), 523. https://doi.org/10.3390/gels9070523
- Tsai, L., Chen, C., Lin, C., Ho, Y., & Mi, F. (2018). Development of mutlifunctional nanoparticles self-assembled from trimethyl chitosan and fucoidan for enhanced oral delivery of insulin. International Journal of Biological Macromolecules, 126, 141–150. https://doi.org/10.1016/j.ijbiomac.2018.12.182
- Urimi, D., Agrawal, A. K., Kushwah, V., & Jain, S. (2019). Polyglutamic acid functionalization of chitosan nanoparticles enhances the therapeutic efficacy of insulin following oral administration. AAPS PharmSciTech, 20(3). https://doi.org/10.1208/s12249-019-1330-2
- Veloso, S. R. S., Azevedo, A. G., Teixeira, P. F., & Fernandes, C. B. P. (2023). Cellulose nanocrystal (CNC) gels: a review. Gels, 9(7), 574. https://doi.org/10.3390/gels9070574
- Wang, A., Yang, T., Fan, W., Yang, Y., Zhu, Q., Guo, S., Zhu, C., Yuan, Y., Zhang, T., & Gan, Y. (2018). Protein corona liposomes achieve efficient oral insulin delivery by overcoming mucus and epithelial barriers. Advanced Healthcare Materials, 8(12). https://doi.org/10.1002/adhm.201801123
- Wang, T., & S, L. (2020). “Oil-soluble” reversed lipid nanoparticles for oral insulin delivery. Journal of Nanobiotechnology, 18, Article 98.
- Wong, C. Y., Al-Salami, H., & Dass, C. R. (2017). Microparticles, microcapsules and microspheres: A review of recent developments and prospects for oral delivery of insulin. International Journal of Pharmaceutics, 537(1–2), 223–244. https://doi.org/10.1016/j.ijpharm.2017.12.036
- Wu, H., Nan, J., Yang, L., Park, H. J., & Li, J. (2022). Insulin-loaded liposomes packaged in alginate hydrogels promote the oral bioavailability of insulin. Journal of Controlled Release, 353, 51–62. https://doi.org/10.1016/j.jconrel.2022.11.032
- Xi, Z., Ahmad, E., Zhang, W., Li, J., Wang, A., Faridoon, Wang, N., Zhu, C., Huang, W., Xu, L., Yu, M., & Gan, Y. (2021). Dual-modified nanoparticles overcome sequential absorption barriers for oral insulin delivery. Journal of Controlled Release, 342, 1–13. https://doi.org/10.1016/j.jconrel.2021.11.045
- Xiao, Y., Tang, Z., Huang, X., Joseph, J., Chen, W., Liu, C., Zhou, J., Kong, N., Joshi, N., Du, J., & Tao, W. (2021). Glucose-responsive oral insulin delivery platform for one treatment a day in diabetes. Matter, 4(10), 3269–3285. https://doi.org/10.1016/j.matt.2021.08.011
- Xu, Y., Zheng, Y., Wu, L., Zhu, X., Zhang, Z., & Huang, Y. (2018). Novel Solid Lipid Nanoparticle with Endosomal Escape Function for Oral Delivery of Insulin. ACS Applied Materials & Interfaces, 10(11), 9315–9324. https://doi.org/10.1021/acsami.8b00507
-
Yin, R., He, J., Bai, M., Huang, C., Wang, K., Zhang, H., Yang, S., & Zhang, W. (2018). Engineering synthetic artificial pancreas using chitosan hydrogels integrated with glucose-responsive microspheres for insulin delivery. Materials Science and Engineering C, 96, 374–382. https://doi.org/10.1016/j.msec.2018.11.032Google Scholar Worldcat Fulltext
- Zhang, H., Wang, W., Li, H., Peng, Y., & Zhang, Z. (2017). Microspheres for the oral delivery of insulin: preparation, evaluation and hypoglycaemic effect in streptozotocin-induced diabetic rats. Drug Development and Industrial Pharmacy, 44(1), 109–115. https://doi.org/10.1080/03639045.2017.1386197
- Zhang, T., Tang, J. Z., Fei, X., Li, Y., Song, Y., Qian, Z., & Peng, Q. (2020). Can nanoparticles and nano‒protein interactions bring a bright future for insulin delivery? Acta Pharmaceutica Sinica B, 11(3), 651–667. https://doi.org/10.1016/j.apsb.2020.08.016
- Zou, J., Wei, G., Xiong, C., Yu, Y., Li, S., Hu, L., Ma, S., & Tian, J. (2022). Efficient oral insulin delivery enabled by transferrin-coated acid-resistant metal-organic framework nanoparticles. Science Advances, 8(8). https://doi.org/10.1126/sciadv.abm4677
Cite this article
-
APA : Saghir, S., Hameed, M., & Kiani, M. T. (2024). Pioneering Oral Insulin Formulations and Delivery Techniques: A Review. Global Pharmaceutical Sciences Review, IX(III), 1-15. https://doi.org/10.31703/gpsr.2024(IX-III).01
-
CHICAGO : Saghir, Savera, Mobina Hameed, and Mahnoor Tariq Kiani. 2024. "Pioneering Oral Insulin Formulations and Delivery Techniques: A Review." Global Pharmaceutical Sciences Review, IX (III): 1-15 doi: 10.31703/gpsr.2024(IX-III).01
-
HARVARD : SAGHIR, S., HAMEED, M. & KIANI, M. T. 2024. Pioneering Oral Insulin Formulations and Delivery Techniques: A Review. Global Pharmaceutical Sciences Review, IX, 1-15.
-
MHRA : Saghir, Savera, Mobina Hameed, and Mahnoor Tariq Kiani. 2024. "Pioneering Oral Insulin Formulations and Delivery Techniques: A Review." Global Pharmaceutical Sciences Review, IX: 1-15
-
MLA : Saghir, Savera, Mobina Hameed, and Mahnoor Tariq Kiani. "Pioneering Oral Insulin Formulations and Delivery Techniques: A Review." Global Pharmaceutical Sciences Review, IX.III (2024): 1-15 Print.
-
OXFORD : Saghir, Savera, Hameed, Mobina, and Kiani, Mahnoor Tariq (2024), "Pioneering Oral Insulin Formulations and Delivery Techniques: A Review", Global Pharmaceutical Sciences Review, IX (III), 1-15
-
TURABIAN : Saghir, Savera, Mobina Hameed, and Mahnoor Tariq Kiani. "Pioneering Oral Insulin Formulations and Delivery Techniques: A Review." Global Pharmaceutical Sciences Review IX, no. III (2024): 1-15. https://doi.org/10.31703/gpsr.2024(IX-III).01
