Abstrict
The oral mucosa offers an attractive route for the systemic delivery of drugs. The bioavailability and effectiveness of many drugs can be increased by administering them through the oral mucosa which bypasses the first-pass metabolism effect. This article will discuss principles, application areas, benefits & drawbacks as well as possible future perspectives for the study and development of oromucosal drug delivery systems. Among the critical benefits of administering drugs via the mouth is that it starts acting fast, is easily absorbed into the bloodstream, makes it easier for patients to follow their prescriptions as well and offers more freedom in terms of dosage control. The article emphasizes innovative technologies such as nanoparticulate formulations as well as microfabricated devices which are slated to improve drug targeting and delivery. It also touches on such related issues as taste masking, variability in bioavailability, and regulatory concerns.
Keywords
Oromucosal Drug Delivery, Bioavailability, Patient Compliance, Drug Targeting, Safety Considerations, Innovative Technologies, Future Directions
Introduction
Drugs applied under the tongue (oromucosal products) are a unique way to deliver drugs through oral mucosa thus increasing their bioavailability and avoiding their first-pass metabolism. These products, which are sprays, gels, or films, as different therapeutic solutions including pain management and treatment for neurological disorders, and the use of oromucosal routes that ensure effective drug delivery.
The possible ways of administrating cannabinoids for medical purposes, one of them being the use of oromucosal products like oromucosal gels and films, is another way of showing how effective and flexible this route of administration is. Acknowledged for their fast action these products aim at both increased patient compliance and decreasing the frequency of administration thereby demonstrating the formidable impact on better patient care as well as outcomes of treatment (Bastos, 2022).
Figure 1

Understanding Oromucosal Products
Oromucosal preparations are designed to deliver drugs through the oral mucosa directly into the bloodstream, bypassing the digestive system, so that rapid treatment can be provided especially when there is an urgent need for intervention. They are found in different forms like pre-filled oral syringes or buccal liquids and are applied against the gums and cheek for better absorption efficiency. Health care practitioners are given the training to be precise with the dosing and the drug expiry dates, they also check for adverse side effects like discomfort, disability, or any emotional problems of the patient, these factors indicate that safety and proper administration techniques are important. Nevertheless, tablets intended for buccal administration are not an outright solution as they may still lead to some unwanted effects like drowsiness, memory loss or even breathing problems, hence close supervision and timely intervention on the part of physicians becomes an indispensable thing. Proper management of drugs after usage has expired or not been used is something to be considered by all of us and this should be the priority (Great, 2021).
Examples of Oromucosal Products
? Buccolam® and Epistatus®: They both have Midazolam Hydrochloride and are primarily used for treating seizures. To enhance absorption, the medication is administered by placing the solution against the sides of the gums and cheek.
? Fentanyl Citrate (Subsys®): It is used for treating breakthrough pain. This drug shows how the oromucosal route can be used for strong medications with dosage control
? Sildenafil Citrate (Viagra®): Showcasing the adaptability of oromucosal products in treating various ailments including erectile dysfunction.
? Buprenorphine and Naloxone (Suboxone®): It is used for the treatment of addiction to opioids in order to show the importance of applying the method of oromucosal routes in medicine (Great, 2021).
Administration and Precautions
When there is an emergency, it is important to follow the right steps in dispensing oromucosal drugs like buccal midazolam, such as verifying the dose and expiry date and giving medication slowly among others. If the attack lasts for over five minutes, the doctor`s instructions should be followed on the administration procedure after confirming the dosage and expiry date of the product. It should be noted that these drugs should be stored at room temperature away from children's reach or anyone else who might misuse them and also ensured that there is medicine available in case anything happens (Great, 2021).
Potential Side Effects
Common adverse reactions associated with midazolam are sleepiness and sedation that are likely to co-occur with memory lapses or short-term forgetfulness. There would be breathing problems while some people also suffered agitation and disorientation problems and became irritable as a result. It is these very products that advantageously serve treatment purposes in different situations because they promote fast-acting and first-pass metabolism evading conditions, that might lead to drug breakdown before reaching its therapeutic parameters (Lingamchetty, 2015).
The popularity of oromuscosal drug administration has greatly increased in recent years an effect that has been influenced by an enhancement in medication assimilation rate compared to the customary oral approach. The multiple benefits of these oromucosal products include passing hepatic first-pass metabolism and pre-systemic degradation. Them appealing pretty much because they are suitable for creating drugs that can serve the purposes of specific patient populations with unmet medical requirements. Significantly, it does not require water making it great for a range of patients like geriatrics, pediatric patients, or uncooperative patients.
Oral patches are extremely valuable during emergency or immediate treatments, as their response time is very fast. The development of pharmaceuticals in this field puts emphasis on patient-focused therapy by adjusting product creation to individual patients' specifics and needs."This innovation covers not only the exploration of novel drug substances but also the reformulation of existing ones and the study of alternative routes of administration in order to improve treatment efficacy, safety, and convenience (Bastos, 2022).
Key Sites for Oromucosal Drug Administration
? Sublingual (Under the Tongue): It is suited for immediate absorption of drugs because the sublingual area rich in blood vessels is the quickest route into the bloodstream through which drugs can enter. For instance, Actair (House Dust Mites Allergen Extract).
? Buccal (Cheek): Appropriate in slower absorbed effective drugs. Drugs get placed between the gums and cheeks to utilize the oral mucosa on the cheeks. For example, Belbuca (Buprenorphine) (Bastos, 2022).
Advantages of Oromucosal Administration
Compounded in terms of bypassing the gastrointestinal tract or avoiding first-pass metabolism in the liver, the oromucosal routes offer various advantages compared to conventional methods. By doing so, an increase in the rate of onset such that it is not only faster but also results in increased availability of the drug which results in greater efficacy. More so, it appeals to many because it offers an option that is not invasive, simple to give, and can be self-administered, mostly those who do not follow instructions when using other methods such as needles or have a fear of drugs administered orally (Zhang, 2002).
Some Advantages of Oromucosal Products Include
Rapid Onset of Action
The numerous blood vessels in the mouth make it easier for drugs to be rapidly absorbed into the bloodstream, resulting in a quicker commencement of action than other administration techniques [8].
Enhanced Bioavailability
Oromucosal delivery of drugs circumvents the alimentary canal and the first metabolization of the drug in the liver increasing the amount of the active ingredient available in the plasma. Hence more potent drug concentrations may be achieved at possibly reduced drug quantity hence better treatment (Masloh, 2023).
Improved Patient Compliance
Patient compliance is greatly improved by the non-intrusive nature of oromucosal products that do not involve needles or pills which can be particularly useful for people with fears or physical problems associated with conventional ones (Rossi, 2005).
Ease of Administration
Drug delivery devices used in the mucosa of the
mouth are easy to use and may be administered by patients themselves, providing a convenient way of taking medicine. Such simple means of application contribute to increased observance on the part of clients and, thus, enhance satisfaction among them (Zhang, 2002).
Flexible Dosing
The systems can be made to allow various amounts for medicines with very small turns of therapeutic difference. Excellent advantages come with the adaptability of the quantity to be used for individual patients without inventing new types of administering the drug.
Site-Specific Targeting
There are specific areas in the oral cavity towards
which some oromucosal delivery systems can be directed. The presence of these oral mucosal targeted delivery systems makes it possible for the treatment to be directed to the specific area without having any systemic effects. It is applicable in situations that necessitate a particular localized action.
Prolonged Residence Time
Certain oromucosal system designs enable longer engagement with the absorption surface, resulting in improved drug absorption and enabling them to be taken less frequently, usually once or twice daily (Madhav, 2009).
Patient Acceptability
Oromucosal films and other vehicles are usually welcomed by patients because they are convenient, portable, and require very little water. Together with possible better taste through taste-masking technologies, these features enhance the probability that they will follow the designated dosage schedule for treatment.
Cost-Effectiveness
Oromucosal drug delivery systems can be developed
and produced at a lower cost compared to other forms because they require less complex manufacturing processes and are characterized by more pocket-friendly means of production yet ensuring that the drug delivery is effective.
Highlighted by these advantages are the chances of oromucosal drug delivery systems making therapeutic outcomes better through improved bioavailability, patient compliance, and convenience.
Challenges in Oromucosal Drug Administration
Despite its benefits, oromucosal administration is not without challenges. The variability in absorption due to the condition of the mucosal membrane, the presence of food or drink, and individual patient differences can affect the
efficacy of the delivered medication. Furthermore, there are limitations in the types of drugs that can be effectively absorbed through this route. Large molecules, for instance, may require special formulation strategies such as the use of penetration enhancers or physical manipulations to facilitate mucosal absorption [6].
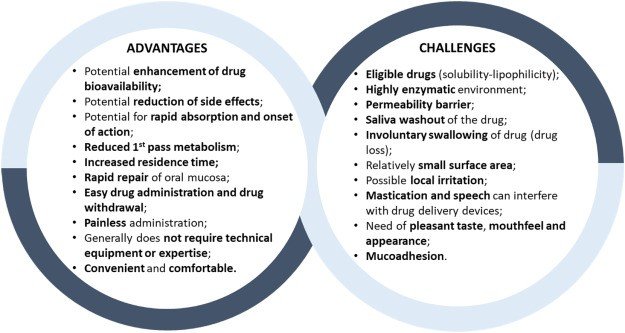
Figure 2
Advantages and Challenges of oromucosal Delivery Site [4,6].
Patient Considerations and Safety
Innovative Technologies in Oromucosal Delivery
Creative technologies in the oral delivery of drugs are moving
fast, promising better targeting of medication and bioavailability. Specifically, nanosized formulations and micro-fabricated systems remain leading in improving accuracy during treatment as well as increasing efficacy.
Nanoparticulate Formulations and Microfabricated Devices
Nanoparticulate formulations like nanoparticles, liposomes, and emulsions, safeguard medicines against harsh gastrointestinal conditions hence increasing their absorption, targeting specific sites, and controlling medication release; likewise, microfabricated devices such as micro containers, patch-like structures or microwells enable one-way drug administration leading to better attachment on intestinal wall as well as improved oral bioavailability (Lou, 2023).
Printing Techniques for Oromucosal Films
Groundbreaking techniques for oromucosal film preparation are being developed such as inkjet printing and roll-to-roll printing which have had their application in this field. It is because of this that they have enabled customization of dose for each patient besides enhancing digitalization and thus safety in personal medicine may be highly enhanced; thus making it easier to administer cure individualized dosages because any extra amount left will not be wasted, unlike in conventional medical treatments. Provided its ability to consider solubility and dissolution process solvent used for making the film has traditionally been cast now it is necessary to complement this technique using innovative printing methods (Jacob, 2023).
Figure 3
Safe Patch Technology
The SAFE Patch is a new innovation in oromucosal drug delivery. A three-layered system comprises - mucoadhesive electrospun nanofibers for high bonding strength, a porous intermediate layer for a large amount of drug, and a saliva-repellent backing film for protection against drug leakage. It functions very well in the oral mucosa extending the life span of the drug and improving its uptake without requiring either liquid or swallowing; hence it is very convenient for the patient (University, 2023).
The utilization of oromucosal delivery will change the way medication administration is done. It enables effective solutions that are more patient-focused and hence ensures increased bioavailability for targeted treatments.
Patient-Centric Features of Oromucosal Films
The primary target for patient-oriented dosage forms are the oromucosal films which are specially designed for cases like those challenging people's lives due to conventional forms of medications. The ability to adhere correctly to mucus membranes is essential for such films as mucoadhesive buccal films (MBFs) in order to enable the effective delivery of drugs, which determines their efficiency.
One way in which orodispersible films can get safer and more traceable is by integrating new, smart components like QR code in them; which completely reduces the chances of getting side effects or consuming substandard prescriptions. This is done via direct printouts on the films that are used to check whether a particular drug is included during drug revision, confirm the safety of consuming those medicines, as well as stop counterfeiters. They are also used to ensure that the dope goes to its owner hence making it specific alongside safeguarding its administration process while at it.
It is of utmost importance to address patient-centric matters particularly when it comes to old cohorts who might have trouble swallowing among other issues. These concerns go a long way in affecting how well oral solid dosage drug delivery systems work. For proper adherence and ultimate patient experience oromucosal films provide an appropriate medium through which medication can be administered conveniently and accurately sticking onto them without any difficulty (Tian, 2019).
Applications in Pain Management
Oromucosal products have been increasingly recognized for their effectiveness in managing various types of pain, particularly through the delivery of medications directly to the bloodstream via the mucous membranes. This section explores the various applications of oromucosal products in pain management, highlighting specific medications and their uses.
Oromucosal Products for Neuropathic Pain
THC: CBD Spray (Nabiximols): Primarily used for
managing chronic neuropathic pain, particularly in conditions such as multiple sclerosis.
Effectiveness in Clinical Trials: Nabiximols have shown significant analgesic efficacy in placebo-controlled clinical trials, especially for MS-associated neuropathic pain.
Long-term Safety and Efficacy: Extended use of nabiximols in chronic pain management has not shown any new safety concerns or evidence of tolerance (Überall, 2020).
Managing Anxiety and Stress in Chronic Pain
Research indicates that anxiety and stress are significant factors in the development and persistence of chronic pain.
Studies suggest the potential benefits of CBD in improving anxiety-related behaviors in neuropathic pain models, which could offer new avenues for pain management strategies (Überall, 2020).
Local Anesthetics for Minor Oral Pain
Pain Relief Gel (Benzocaine): Used for short-term relief from pain caused by minor mouth issues such as toothaches and sore gums.
Application Instructions: It is advised to apply the gel to the affected area and leave it for at least one minute before rinsing, with a maximum of four applications per day.
Over-the-counter solutions for Sore Throat and Mouth
Phenol Sprays and Suspensions: These are accessible OTC solutions for relieving pain and irritation from sore throats and canker sores.
Nonsteroidal Anti-Inflammatory Drugs (NSAIDs) for Dental Pain
Efficacy in Dental Pain: NSAIDs are preferred over opioids for managing acute dental pain due to their effectiveness in reducing inflammation-related pain.
Mechanism of Action: These drugs inhibit the cyclooxygenase enzyme, crucial for prostaglandin production, which plays a key role in mediating pain and inflammation.
Combination Therapies
NSAIDs and Acetaminophen: These medications can be used together to effectively reduce mild to moderate pain, leveraging their distinct mechanisms of action.
This overview of oromucosal applications in pain management illustrates the versatility and effectiveness of these products in addressing different types of pain, from acute dental pain to chronic neuropathic conditions. Their ability to deliver medication efficiently and bypass gastrointestinal metabolism makes them an invaluable tool in the pain management arsenal (Oral, 2020).
Challenges and Limitations
Variability in Bioavailability and Formulation Challenges
Diversity in mucosal health in every individual or other differences in the rate of saliva production can change bioavailability and will pose some hindrance to oromucosal drug delivery. Other challenges facing these drug formulations include formulation problems since these drugs require highly specific preparations in order to achieve good absorption when applied to a small region.
Biological and Technical Barriers
The thick viscous substance forms a vital hindrance on the way of drugs' crossing into the submucosa, thus affecting absorption efficacy. Besides, within an organism, the gut lumen also plays a significant role because its contents can change the shape of an active drug molecule in the tissues. For example, making pharmacological preparations intended for ingestion might be difficult due to the fact that scientists have not yet found ways out concerning the issues faced by biological barriers. Another problem is associated with the development of delivery systems like patches on intestine walls or needles to solve these problems.
Taste Masking and Stability Issues
It is very important because undesirable tastes can result in patients not taking their medication as prescribed. The drug must remain stable both in storage and when used to avoid breakdown so that the desired level of effectiveness is maintained" (Masloh, 2023).
Limited Drug Suitability and Systemic Challenges
There are constraints to the transmucosal route that allow only a few drugs in because the fluid volume is too small to dissolve many drugs and a mucus border prevents most drugs from dissolving. Thus, the types of diseases that can easily be treated effectively via oromucosal means are narrowed down. Apart from that, it is important to manage other matters such as fast dissolution rates and local toxicology in order to prevent side effects.
Scaling-Up and Commercialization
Oromucosal delivery system scaling for commercial manufacture faces difficulty in holding up quality, safety, and efficacy. There must be careful and controlled planning at every level during the scale-up process to see that the final product is in line with the regulations.
Enzymatic Degradation and Poor Tissue Penetration
There are occurrences where the effectiveness of delivered medication can be reduced dramatically because of enzymes that degrade them within the mouth cavity. To compound problems further, penetration of tissue is minimal and there is a real danger that you may swallow it mistakenly when taken orally hence there is a need for formulation strategies that are more advanced to tackle these challenges.
Regulatory Considerations for Oromucosal Drug Approval
One way to preserve the exact wording and HTML elements while making it sound more like a human wrote it is by Outlining a text that makes it easier to understand and removing complicated phrases since they reduce burstiness and make it easy for a reader to go through the entire text. This is an example: Regulatory oversight of oromucosal drugs is an important role that the U.S. Food & Drug Administration (FDA) plays in ensuring their stringent safety and efficacy standards are met before they become available in the market. The various steps that these products have to pass through are crucial as each of them aims at investigating thoroughly its effect on patient health" (Krampe, 2016; Cilurzo, 2017; Xu, 2024).
FDA Regulatory Framework
The regulatory framework for oromucosal medications of the FDA is specified in Title 21 of the Code of Federal Regulations (CFR Title 21) which is updated annually and accessible via the Electronic Code Of Federal Regulations (eCFR) website w1645e. This complete group of principles embraces walls from drug conception through post-market surveillance giving assurance that each detail relating to drug safety and efficiency is critically reviewed (Krampe 2016; Cilurzo 2017; Xu, 2024).
Preclinical and Clinical Requirements
Preclinical studies involve investigating how a drug is taken up, how it works in the body, and what effect it has on the body. They are done using cells in test tubes or animals like rats or rabbits..decide how much drug is safe and what some of its harmful effects could be. If the preclinical testing is successful, the drug is tried out on humans, usually in three separate stages in order to examine its safety, effectiveness, dosing, and side effects (Krampe 2016; Cilurzo 2017; Xu 2024).
Submission and Review Process
The pre-clinical and clinical trials have to be done to determine whether this oromucosal medication is actually safe and effective before any marketing applications are made such as submitting a New Drug Application (NDA) or requesting a Marketing Authorization Application (MAA) to the FDA or EMA. The FDA and EMA review these submissions, often consult external experts, or ask for more information & change labels. These applications must include detailed data on drug chemistry, manufacturing process, and proposed labeling, as well as safety updates as well as an assessment of abuse potential (Krampe 2016; Cilurzo 2017; Xu, 2024).
Post-Approval Obligations
Just because a sponsor receives approval for an oromucosal drug format, does not mean their job is finished in terms of regulation. Post-approval obligations comprise continuous pharmacovigilance programs, regular safety update reports, and probable product information changes according to fresh data concerning safety and effectiveness (PIMS, 2004). Moreover, it is necessary that the medicine should adhere to some specific requirements regarding its name tags and packaging for risk minimization concerning misuse, abuse, or accidental exposure.
Special Regulatory Pathways
The FDA has created additional regulatory pathways to
accelerate approval for oromucosal drugs that are intended to treat severe conditions. These pathways include accelerated approval on surrogate endpoints, urgent research designation, and fast-track status. After all, the medications must comply with all relevant safety and efficacy standards for the review process to be hastened as well. (Krampe 2016; Cilurzo 2017; Xu 2024).
Manufacturing and Clinical Practice Standards
The regulatory agencies mandate that for an
oromucosal agent to be safe and effective, adherence to both GMPs and GCPs must be maintained during the drug development and approval process. Adherence to these standards maintains the drug's quality and reliability during manufacture and clinical trials (Moini, 2023)."
A complex yet significant process is navigating the regulatory landscape for oromucosal drug authorization, this guarantees effective medication safety and its use by the population. Adherence to these strict criteria allows pharmaceutical companies to introduce new oromucosal products into the market creating key treatment options for patients.
Future Directions and Potential
There is much promise for a better tomorrow in medicine due to modern technological advancements that have taken place, leading to the emergence of a more sophisticated way of thinking concerning the administration of medicines. What follows is a list containing items that could form agenda items of further investigations concerned with future orientations concerning such issues—as they relate to pharmacological aspects within healthcare delivery systems. What will follow next are some indicative areas on which future studies and development targets need to be focused.
Natural Compounds and Their Therapeutic Effects
Honey, Aloe Vera, Curcumin, and Propolis: Scientists are studying these chemicals because they may help to stop and cure oral mucositis among patients diagnosed with cancer, representing drug reformulation for the management of a painful condition.
Royal Jelly: Royal jelly has gained prominence because of the many ways it is believed to improve health. Plus it has the potential to repair the damage done to the mouth due to drugs at 3% concentration, 10% concentration, and even up to 30% concentration levels (ROYAL, 2020).
Hydroethanolic Extract of S. mombin Leaves: In studies of laboratory animals, this section proved promising in the reduction of oxidative stress and inflammation that accelerates the healing of mouth sores arising from chemotherapy (Ferreira, 2020).
Oromucosal Administration of Oxytocin
Oromucosal delivery of oxytocin presentation is a promising strategy, especially in pediatrics and geriatrics where it offers better tolerability than other routes and hence compliance in long-term use (Xu, 2024).
Advanced Drug Delivery Systems
The Efficacy and Safety of Oromucosal Products will be improved by Drug Delivery Innovations as:
Nanoparticle-Based Systems: These systems may improve drug targeting and bioavailability.
Stimuli-Responsive Drug Delivery Systems and Smart Polymers: These processes create an opportunity for precision in administering medicine by enabling a reserved discharge based on specific causes.
Microelectromechanical Systems (MEMS): MEMS finds utility in inaccurate drug tonic dispensation and hence can ability in boosting the capabilities of oramucosal products even further.
Enhancements in Oral Dosage Forms
Drug manufacturers made an innovation that could solve problems connected to drug intake and use helping to make available the best service for patients and efficient treatment delivery.
The Role of Blood Supply in Drug Bioavailability
Highly vascularized mucosal surfaces with abundant blood supply significantly contribute to the quick movement of medications into the bloodstream, a process that is necessary for evading liver metabolism during the first passage and augmenting how well drugs work (Madhav, 2009).
Expanding the Scope of Orotansmucosal Routes
In exploring future oromucosal products, a wider range of orotransmucosal routes for delivering medicine can be explored. Successes achieved recently in this field provide the impetus behind this growth in addition to ongoing achievements therefore demonstrating how it allows for various types of therapies that are flexible but efficient.
They underscore the increasing significance of oromucosal drug delivery systems in contemporary medical practice, as well as emphasizing the continuing progression of these areas of study. Accessing diverse health applications, these areas can revolutionize drug administration including patients’ experiences.
Conclusion
We have talked about the complex world of oromucosal drug delivery in this paper and the principles, merits, and cutting-edge techniques that could change the face of medication administration. Oromucosal products have shown great promise in providing more efficient, patient--
focused treatment options for various diseases, ranging from the improvement in bioavailability and patient adherence to the side-stepping of traditional drug delivery challenges. There are significant opportunities for advancing patient-centered treatments through the use of sublingual medications, with breakthroughs in nanotechnology and microencapsulation making it possible to administer drugs with no need for organic solvents.
The future clearly shows us that the quest and growth of oromucosal medication distribution systems will continue to significantly improve healthcare. Both emphases on patient-centered resolutions and the untiring attempt at enhancing bioavailability and curative effectiveness reveal the transformation capability of those provision modalities. If current limitations are targeted and instead, inventive technologies are used, the potential for powders. The possibilities for oromucosal products to enhance treatment results and patient experience are limitless, indicating a thrilling new chapter in drug delivery owing to overcoming contemporary setbacks as well as utilizing modern technologies.
Figure 3

References
-
Bastos, F., Pinto, A. R., Nuñes, A. J. A., & Simões, S. (2022). Oromucosal products – Market landscape and innovative technologies: A review. Journal of Controlled Release, 348, 305–320. https://doi.org/10.1016/j.jconrel.2022.05.053
-
Great Ormond Street Hospital. (n.d.). Buccal (oromucosal) midazolam. GOSH Hospital Site. https://www.gosh.nhs.uk/conditions-and-treatments/medicines-information/buccal-oromucosal-midazolam/
- Lingamchetty TN, Hosseini SA, Saadabadi A. Midazolam. (2023). In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-. https://www.ncbi.nlm.nih.gov/books/NBK537321/
-
Bastos, F., Pinto, A. R., Nuñes, A. J. A., & Simões, S. (2022b). Oromucosal products – Market landscape and innovative technologies: A review. Journal of Controlled Release, 348, 305–320. https://doi.org/10.1016/j.jconrel.2022.05.053
- Zhang, H., Zhang, J., & Streisand, J. B. (2002). Oral mucosal drug delivery. Clinical Pharmacokinetics, 41(9), 661–680. https://doi.org/10.2165/00003088-200241090-00003
-
Phenol (Oromucosal route) proper use - Mayo Clinic. (n.d.). https://www.mayoclinic.org/drugs-supplements/phenol-oromucosal-route/proper-use/drg-20072736?p=1
- Masloh S, Culot M, Gosselet F, Chevrel A, Scapozza L, Zeisser Labouebe M. Challenges and Opportunities in the Oral Delivery of Recombinant Biologics. Pharmaceutics. 515(5), 1415. https://doi.org/10.3390/pharmaceutics15051415.
- Rossi, S., Sandri, G., & Caramella, C. (2005). Buccal drug delivery: A challenge already won? Drug Discovery Today. Technologies, 2(1), 59–65. https://doi.org/10.1016/j.ddtec.2005.05.018
- Madhav, N. V. S., Shakya, A. K., Shakya, P., & Singh, K. (2009). Orotransmucosal drug delivery systems: A review. Journal of Controlled Release, 140(1), 2–11. https://doi.org/10.1016/j.jconrel.2009.07.016
- Tian, Y., Orlu, M., Woerdenbag, H. J., Scarpa, M., Kiefer, O., Kottke, D., & Visser, J. C. (2019). Oromucosal films: from patient centricity to production by printing techniques. Expert Opinion on Drug Delivery, 16(9), 981–993. https://doi.org/10.1080/17425247.2019.1652595
- Lou J, Duan H, Qin Q, Teng Z, Gan F, Zhou X, Zhou X. Advances in Oral Drug Delivery Systems: Challenges and Opportunities. Pharmaceutics. 15(2), 484. https://doi.org/10.3390/pharmaceutics15020484.
- Jacob, S., Boddu, S. H. S., Bhandare, R. R., Ahmad, S., & Nair, A. B. (2023). Orodispersible Films: current innovations and emerging trends. Pharmaceutics, 15(12), 2753. https://doi.org/10.3390/pharmaceutics15122753
- University of Copenhagen. (2023). A more efficient technology for oromucosal drug delivery. https://pharmacy.ku.dk/news/news-2023/a-more-efficient-technology-for-oromucosal-drug-delivery/
- Tian, Y., Orlu, M., Woerdenbag, H. J., Scarpa, M., Kiefer, O., Kottke, D., Sjöholm, E., Öblom, H., Sandler, N., Hinrichs, W. L. J., Frijlink, H. W., Breitkreutz, J., & Visser, J. (2019). Oromucosal films: from patient centricity to production by printing techniques. Expert Opinion on Drug Delivery, 16(9), 981–993. https://doi.org/10.1080/17425247.2019.1652595
- Überall MA. A Review of Scientific Evidence for THC: CBD Oromucosal Spray (Nabiximols) in the Management of Chronic Pain. J Pain Res. 13, 399-410. https://doi.org/10.2147/JPR.S240011.
- Oral analgesics for acute dental pain. (n.d.-b). American Dental Association. https://www.ada.org/en/resources/ada-library/oral-health-topics/oral-analgesics-for-acute-dental-pain
- Krampe, R., Visser, J.C., Frijlink, H.W., Breitkreutz, J., Woerdenbag, H.J., & Preis, M. (2016). Oromucosal film preparations: points to consider for patient centricity and manufacturing processes. Expert Opinion on Drug Delivery, 13, 493 - 506.
- Cilurzo, F., Musazzi, U.M., Franzè, S., Selmin, F., & Minghetti, P. (2017). Orodispersible dosage forms: biopharmaceutical improvements and regulatory requirements. Drug Discovery Today, 23 2, 251-259.
- Ferreira AS, Macedo C, Silva AM, Delerue-Matos C, Costa P, Rodrigues F. Natural Products for the Prevention and Treatment of Oral Mucositis-A Review. Int J Mol Sci. 23(8):4385. https://doi.org/10.3390/ijms23084385.
- Xu, D., Lan, C., Kou, J., Yao, S., Becker, B., & Kendrick, K. M. (2024). Oromucosal administration of oxytocin: the development of ‘Oxipops.’ Pharmaceutics, 16(3), 333. https://doi.org/10.3390/pharmaceutics16030333
- Madhav, N. V. S., Shakya, A. K., Shakya, P., & Singh, K. (2009b). Orotransmucosal drug delivery systems: A review. Journal of Controlled Release, 140(1), 2–11. https://doi.org/10.1016/j.jconrel.2009.07.016
- Moini, J., LoGalbo, A., & Schnellmann, J. G. (2023). Drug approval and drug regulations. In Elsevier eBooks (pp. 13–28). https://doi.org/10.1016/b978-0-323-95974-2.00031-1
-
Bastos, F., Pinto, A. R., Nuñes, A. J. A., & Simões, S. (2022). Oromucosal products – Market landscape and innovative technologies: A review. Journal of Controlled Release, 348, 305–320. https://doi.org/10.1016/j.jconrel.2022.05.053
-
Great Ormond Street Hospital. (n.d.). Buccal (oromucosal) midazolam. GOSH Hospital Site. https://www.gosh.nhs.uk/conditions-and-treatments/medicines-information/buccal-oromucosal-midazolam/
- Lingamchetty TN, Hosseini SA, Saadabadi A. Midazolam. (2023). In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-. https://www.ncbi.nlm.nih.gov/books/NBK537321/
-
Bastos, F., Pinto, A. R., Nuñes, A. J. A., & Simões, S. (2022b). Oromucosal products – Market landscape and innovative technologies: A review. Journal of Controlled Release, 348, 305–320. https://doi.org/10.1016/j.jconrel.2022.05.053
- Zhang, H., Zhang, J., & Streisand, J. B. (2002). Oral mucosal drug delivery. Clinical Pharmacokinetics, 41(9), 661–680. https://doi.org/10.2165/00003088-200241090-00003
-
Phenol (Oromucosal route) proper use - Mayo Clinic. (n.d.). https://www.mayoclinic.org/drugs-supplements/phenol-oromucosal-route/proper-use/drg-20072736?p=1
- Masloh S, Culot M, Gosselet F, Chevrel A, Scapozza L, Zeisser Labouebe M. Challenges and Opportunities in the Oral Delivery of Recombinant Biologics. Pharmaceutics. 515(5), 1415. https://doi.org/10.3390/pharmaceutics15051415.
- Rossi, S., Sandri, G., & Caramella, C. (2005). Buccal drug delivery: A challenge already won? Drug Discovery Today. Technologies, 2(1), 59–65. https://doi.org/10.1016/j.ddtec.2005.05.018
- Madhav, N. V. S., Shakya, A. K., Shakya, P., & Singh, K. (2009). Orotransmucosal drug delivery systems: A review. Journal of Controlled Release, 140(1), 2–11. https://doi.org/10.1016/j.jconrel.2009.07.016
- Tian, Y., Orlu, M., Woerdenbag, H. J., Scarpa, M., Kiefer, O., Kottke, D., & Visser, J. C. (2019). Oromucosal films: from patient centricity to production by printing techniques. Expert Opinion on Drug Delivery, 16(9), 981–993. https://doi.org/10.1080/17425247.2019.1652595
- Lou J, Duan H, Qin Q, Teng Z, Gan F, Zhou X, Zhou X. Advances in Oral Drug Delivery Systems: Challenges and Opportunities. Pharmaceutics. 15(2), 484. https://doi.org/10.3390/pharmaceutics15020484.
- Jacob, S., Boddu, S. H. S., Bhandare, R. R., Ahmad, S., & Nair, A. B. (2023). Orodispersible Films: current innovations and emerging trends. Pharmaceutics, 15(12), 2753. https://doi.org/10.3390/pharmaceutics15122753
- University of Copenhagen. (2023). A more efficient technology for oromucosal drug delivery. https://pharmacy.ku.dk/news/news-2023/a-more-efficient-technology-for-oromucosal-drug-delivery/
- Tian, Y., Orlu, M., Woerdenbag, H. J., Scarpa, M., Kiefer, O., Kottke, D., Sjöholm, E., Öblom, H., Sandler, N., Hinrichs, W. L. J., Frijlink, H. W., Breitkreutz, J., & Visser, J. (2019). Oromucosal films: from patient centricity to production by printing techniques. Expert Opinion on Drug Delivery, 16(9), 981–993. https://doi.org/10.1080/17425247.2019.1652595
- Überall MA. A Review of Scientific Evidence for THC: CBD Oromucosal Spray (Nabiximols) in the Management of Chronic Pain. J Pain Res. 13, 399-410. https://doi.org/10.2147/JPR.S240011.
- Oral analgesics for acute dental pain. (n.d.-b). American Dental Association. https://www.ada.org/en/resources/ada-library/oral-health-topics/oral-analgesics-for-acute-dental-pain
- Krampe, R., Visser, J.C., Frijlink, H.W., Breitkreutz, J., Woerdenbag, H.J., & Preis, M. (2016). Oromucosal film preparations: points to consider for patient centricity and manufacturing processes. Expert Opinion on Drug Delivery, 13, 493 - 506.
- Cilurzo, F., Musazzi, U.M., Franzè, S., Selmin, F., & Minghetti, P. (2017). Orodispersible dosage forms: biopharmaceutical improvements and regulatory requirements. Drug Discovery Today, 23 2, 251-259.
- Ferreira AS, Macedo C, Silva AM, Delerue-Matos C, Costa P, Rodrigues F. Natural Products for the Prevention and Treatment of Oral Mucositis-A Review. Int J Mol Sci. 23(8):4385. https://doi.org/10.3390/ijms23084385.
- Xu, D., Lan, C., Kou, J., Yao, S., Becker, B., & Kendrick, K. M. (2024). Oromucosal administration of oxytocin: the development of ‘Oxipops.’ Pharmaceutics, 16(3), 333. https://doi.org/10.3390/pharmaceutics16030333
- Madhav, N. V. S., Shakya, A. K., Shakya, P., & Singh, K. (2009b). Orotransmucosal drug delivery systems: A review. Journal of Controlled Release, 140(1), 2–11. https://doi.org/10.1016/j.jconrel.2009.07.016
- Moini, J., LoGalbo, A., & Schnellmann, J. G. (2023). Drug approval and drug regulations. In Elsevier eBooks (pp. 13–28). https://doi.org/10.1016/b978-0-323-95974-2.00031-1
Cite this article
-
APA : Talha, S. M., Zainab, P., & Khalid, M. (2023). Enhancing Bioavailability with Oromucosal Products: Opportunities and Challenges. Global Pharmaceutical Sciences Review, VIII(IV), 37-48. https://doi.org/10.31703/gpsr.2023(VIII-IV).04
-
CHICAGO : Talha, Syed Muhammad, Pakeeza Zainab, and Mamoona Khalid. 2023. "Enhancing Bioavailability with Oromucosal Products: Opportunities and Challenges." Global Pharmaceutical Sciences Review, VIII (IV): 37-48 doi: 10.31703/gpsr.2023(VIII-IV).04
-
HARVARD : TALHA, S. M., ZAINAB, P. & KHALID, M. 2023. Enhancing Bioavailability with Oromucosal Products: Opportunities and Challenges. Global Pharmaceutical Sciences Review, VIII, 37-48.
-
MHRA : Talha, Syed Muhammad, Pakeeza Zainab, and Mamoona Khalid. 2023. "Enhancing Bioavailability with Oromucosal Products: Opportunities and Challenges." Global Pharmaceutical Sciences Review, VIII: 37-48
-
MLA : Talha, Syed Muhammad, Pakeeza Zainab, and Mamoona Khalid. "Enhancing Bioavailability with Oromucosal Products: Opportunities and Challenges." Global Pharmaceutical Sciences Review, VIII.IV (2023): 37-48 Print.
-
OXFORD : Talha, Syed Muhammad, Zainab, Pakeeza, and Khalid, Mamoona (2023), "Enhancing Bioavailability with Oromucosal Products: Opportunities and Challenges", Global Pharmaceutical Sciences Review, VIII (IV), 37-48
-
TURABIAN : Talha, Syed Muhammad, Pakeeza Zainab, and Mamoona Khalid. "Enhancing Bioavailability with Oromucosal Products: Opportunities and Challenges." Global Pharmaceutical Sciences Review VIII, no. IV (2023): 37-48. https://doi.org/10.31703/gpsr.2023(VIII-IV).04
