02 Pages : 7-18
Abstrict
Using the capsule as a dosage form dates back to the early pharmacy days; from those days, the capsule preparation process is greatly evolved in order to meet the current needs of patients and the pharmaceutical industry. Currently, all pharmaceutical industries are making a dynamic change in the specifications of particular dosage forms such as capsules. It has become more difficult to obtain the higher valued drug product from the dosage form, along with the potential for improved delivery that can improve clinical outcomes. In addition, the capsules have played a significant role in drug development because of the hand filling or the production of semiautomatic capsular formulations, which have fewer requirements, and can be developed easily. The article will discuss different tests like Universal tests and specific tests, particularly for capsules, performed prior to the release of the batch to ensure efficacy and safety.
Keywords
Capsule, Specifications, Dosage Form, Hand Filling, Semiautomatic Capsular Formulations, Universal Tests and Specific Tests.
Introduction
Capsules are the most versatile form of all the dosage forms. They are a type of solid dosage forms in which more than one medicinal (API) and inert(excipients) are enclosed in the small gelatin shell container. Dry powders, semi-solids, and liquids that do not dissolve gelatin may be encapsulated. Capsules account for about 20% of all prescriptions dispensed. (Withey & Mainville, 1969)
Two main types of capsules include:
• The Hard Gelatin Capsule
• The Soft Gelatin Capsule
Other types include:
• Modified-Release capsule
They are further divided into:
• Sustained-release Capsule (Prolonged and the extended-release capsule)
• Delayed-release Capsule (Enteric-coated capsules)
Characterization of Capsules
It refers to the features or the characteristics regarding the capsules bear on its intrinsic ability in order to satisfy the need of the consumers. Features like shape, colour, dimension, composition, disintegration, dissolution, filling etc., are assured to do the characterization of capsules.
For this purpose, different Quality Control Tests are performed. Characterization of capsules is done to assure:
1. Efficacy
2. Safety
3. Quality
4. Compliance
Quality Control in the Pharmaceutical Industry
It is an important function of the pharmaceutical industry. The drugs should be marked as therapeutic and safe types, and their effectiveness is predictable and usually consistent. Better medical devices are now being developed at a faster rate. At the same time, advanced analytical methods are designed for their testing. (Taylor, Ginsburg, Hickey, & Gheyas, 2000)
Tests for the Characterization of Capsule Preparation
Whether the capsules are manufactured in small quantities or in large quantities, they all need to pass a specific test for e.g. a QC test, to assess the quality of the finished products.
These test fall into two categories:
1. Universal tests
2. Specific tests for the specific dosage form
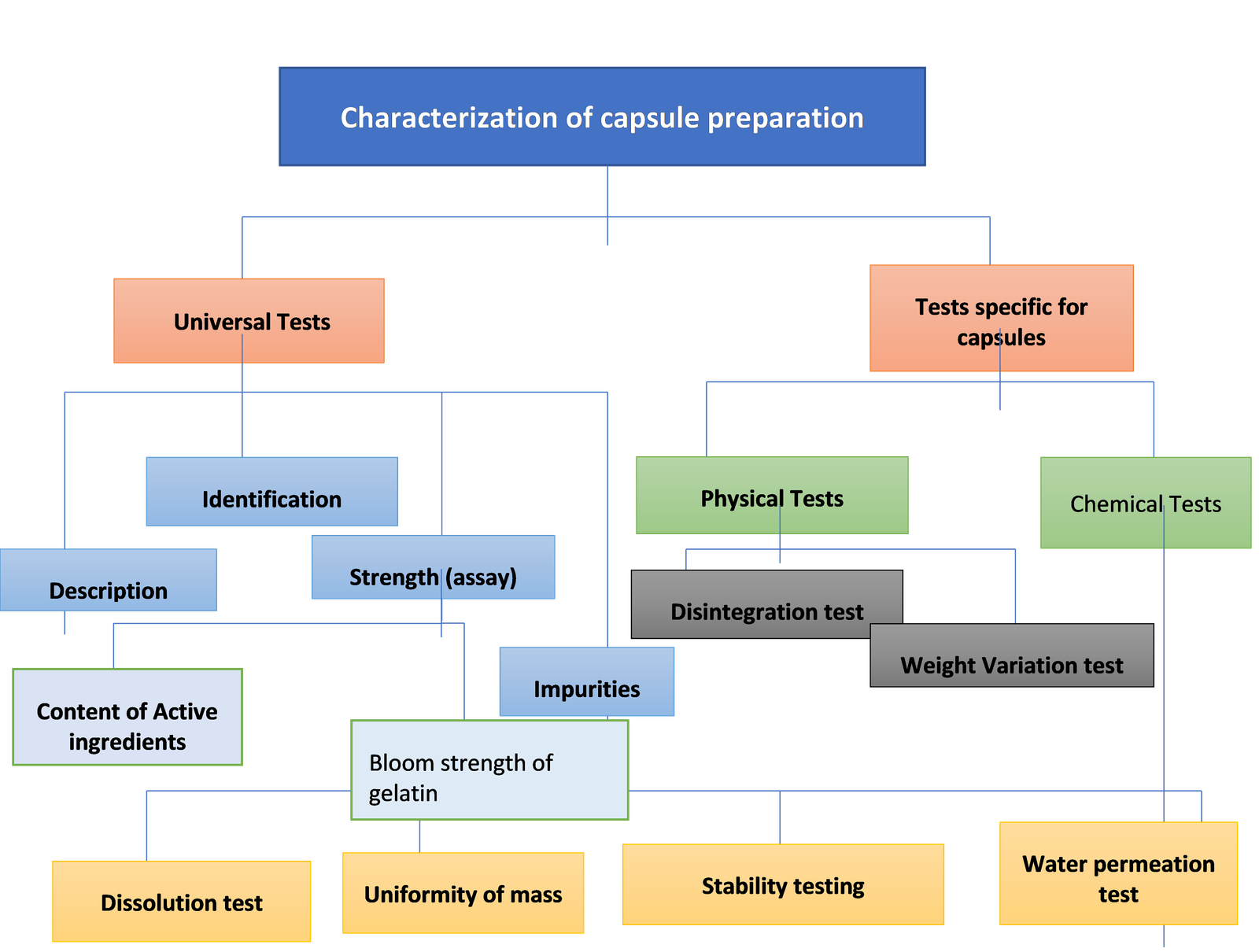
Universal Tests
These tests are common and are referred to as Generalized Tests for all oral forms of dosage. These are essential to assure that the products which are commercialized meet the minimum requirements of quality.
These tests include:
A. Description of dosage form
B. Identification of dosage form
C. Strength that includes assay test
D. Impurities, either organic, inorganic or residual solvents
Description
It is not known to be a standard; rather, it is categorized as general in nature. It usually communicates well with the appearance of an entity
and complies with the monograph standards. We usually inspect and unpack at least 20 capsules.
Appearance
• They should have a smooth surface and non-abrasive.
• The very first evidence of the instability is indicated as significant changes, which may be in the body shape of the capsule along with the firmness or softening, cracking of capsule due to external force, swelling, bending, or discolouration of the capsular shell.
• Capsules are manufactured on a small as well as on a large scale, so they all should be uniform in appearance.
Shape and Size
• The hard gelatin capsules consist of a range
of different sizes, one of the standard ones at the industrial level is employed for the human medicines, which have a size range from 000 designated as the largest (1.40ml), up to 5, which is the smallest (0.13ml) are still commercially available.
Table 1. Capsule sizes and volume capacity
|
Empty Capsule Size |
||||||||||
|
Capsule Size |
000 |
00E |
00 |
0E |
0 |
1 |
2 |
3 |
4 |
5 |
|
Empty Capsule Volume Capacity (ml) |
||||||||||
|
Capacity |
1.37 |
1.00 |
0.90 |
0.78 |
0.68 |
0.48 |
0.36 |
0.27 |
0.20 |
0.13 |
• Soft gelatin capsule is available in different shapes such as sperical (0.04-5ml) , ovoid shape (0.06-7ml), cylindrical shape(0.15-25ml), tube-like (0.5-0ml), pear-shaped (0.3-5ml) etc.
Diameter
A capsular diameter sorter is the one that allows it to pass through the coming unit of a capsule which should be within range of + or - 0.020 inch (theoretical diameter).
Color
The capsule is fed automatically by using a
Pneumatic conveyer with the help of a diameter sorter. In regard to this particular unit, capsules whose color do not match the reference colour, standard solution of the product is discarded while others pass the colour test. (Hsieh, 1994; Samyn & Jung, 1970)
Composition of the shells
• A hard gelatin capsule is a kind of capsule mainly composed of gelatin and water. Some colored hard gelatin capsules also contain a considerable quantity of Titanium dioxide and other colorants. The following table presents a particular composition.
Table 2. Composition of hard gelatin capsule shells
|
Name |
Gelatin |
Water |
Titanium dioxide |
Colorant |
|
Transparent |
85% |
15% |
/ |
/ |
|
Opaque |
82-84% |
16% |
0.4-1.8% |
0.3-0.5% |
• Soft gelatin capsules have similar composition as hard gelatin capsules, except that there is a difference in the moisture proportion. Mostly there is the addition of plasticizers to allow the capsules’ stability and elasticity for e.g. Glycerol and Sorbitol etc. it can be filled with liquid as it is a one-piece capsule. (Fatohy; Galia et al., 1998)
B.Identification
It is mentioned in the monograph as an aid for the confirmation that the article contains the labelled drug substance by providing the positive identification of drug entity in the drug product. The analytical procedure must be able to distinguish the active ingredient from all excipients or from potential degradation products likely to be present.
General tests performed to identify the active ingredient in the capsules are:
• Thin-layer Chromatographic Identification
• Spectrophotometric Identification tests
• Nuclear Magnetic Resonance etc.
Thin-Layer Chromatographic Identification Test
Method
Prepare the test solution as directed at each individual monograph. In a line about 2cm from the edges of the chromatographic plate, covered with 0.24mm layer of silica gel chromatographic mixture, insert 10 ml of the solution, 10 ml of the standard solution USP, which is the Reference Standard in case of a particular drug substance, to be identified in the same solvent, at the same time, otherwise mentioned in the individual monograph. Allow the stains to dry and the chromatogram to be developed in a solvent system comprising of a mixture containing chloroform, water and methanol unless the solvent front occupies three-quarters of plate length. Mark only the front mark. Find spots on a plate while testing it under a short UV lamp.
Interpretation
The value of Rf in the main area obtained in the test solution corresponds to that found in the standard solution.
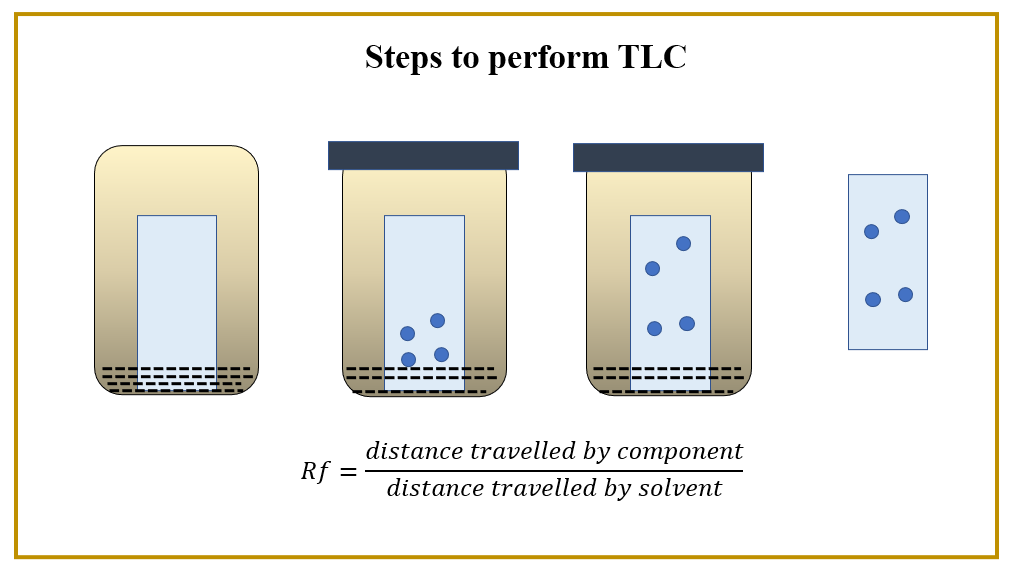
Figure 1: Steps of Thin-layer Chromatographic Identification Testing
Spectrophotometric Identification Test
These experiments contribute to the identification of many chemical compounds. The following diagnostic procedures apply to IR or to UV radiation. The spectrum of infra-Red absorption as compared to that obtained in accordance with the corresponding Reference Standard USP perhaps provides the most compelling evidence of substance identity that can be found in any single test. On the other hand, the UV absorption lines do not show high specificity.
Infrared Absorption
Method
Record the test sample line and the corresponding Reference Standard USP in the range from 2.6µm-15µm (3800 cm-1-650cm-1), otherwise quoted in the monograph.
Interpretation
The IR description of the test sample shows only maxima at the same wavelengths as the corresponding Reference Standard of USP.
Ultraviolet Absorption
Method
Complete part of the subject under test in the selected medium to obtain the test solution with the concentration specified in the monograph for a
solution. On a similar basis, make a standard solution having the same Reference Standard USP. Compare spectra at a spectral range of 200-400nm unless otherwise specified for each person given a test solution and standard solution. Calculate the input and absorption values when these methods are included in a single monograph.
Interpretation
Requirements are met if UV absorption of the test solution and standard soln. Show the maxima and minima at the same wavelengths as the acquisition and absorption values within the set limits.
Nuclear Magnetic Resonance
Although widely recognized as one of the most powerful structure-elucidation tools available, with proper experimental design, it can also be used for accurate qualitative and quantitative measurements. (Chiwele, Jones, Podczeck, & Bulletin, 2000)
Internal Reference Standard
The reference standard is co-dissolved in the analyte test sol.
Typical NMR solution preparation
An NMR solution is prepared with exact weights of both the analyte and reference standard. To minimize the error, use larger weights.
Interpretation
The quantitative method is based on a comparison of the reference standard and analyte NMR peaks and their respective conc. Process the data using zero-filling if necessary, such that a sufficient no. of points define a peak. Integrate appropriate peaks which give the result quantitatively, and it should be in the range given in the individual monograph.
Strength (essay test)
The assay is the specific and stability-indicating test in order to find the potency (content) of the capsules. When non-specific assay for, e.g., titration is justified,
all other analytical procedures ensure the absence of any interfering species. It is sometimes called a content test.
Active Ingredients (API)Content
Method
In this method, the sample content is tested as mentioned in the individual monograms, and the calculated amount of the active ingredient in each of the capsule is determined. According to BP, the content of the
active ingredient mentioned in the monograph is required 20 capsules.
Table 3. Active ingredients(API) Limits
|
Weight of API in capsule (g) |
Subtract (lower
limit) |
Addition (upper
limit) |
|
|
14
11 5 |
16
11 5 |
|
0.13 or less |
0.3
0.6 |
0.3
0.7 |
|
More than 0.13 Less than 0.4 |
0.3
0.5 1.3 |
0.4
0.5 1.6 |
|
0.4 0r more |
0.2
0.3 0.7 |
0.4
0.5 1.1 |
Acceptance criteria The preparation complies with the test if not more than one individual content is outside the limits of 85-115% of the average content and none is outside the limits of 75-125% of
Acceptance criteria
The preparation complies with the test if not more than one individual content is outside the limits of 85-115% of the average content and none is outside the limits of 75-125% of the average content.
Bloom Strength of the Gelatin
The capsule shell gelatin should be tested for various body components such as blooming strength and the viscosity.
Method
Gelatin is mixed in water to make 6.57% solution in conventional bloom bottles. Mixture is stirred then stored at room temperature for three hours. The bottles were then transferred to the 65 degrees bath, for time period of 20 minutes. Let the bloom pots or jars cool down for 15 minutes in a temporary room. They are then prepared or conditioned for 16hrs in water bath. When performing a bloom gelatin test, bloom pot is focussed along with probe placed just above the sample being used.
Interpretation
Penetration of the probe in gelatin at the targeted depth of 3mm with speed 0.4mm / s and then recedes. Higher force is strength of the gel in unit grams.
D. Impurities
Process contaminants, synthetic products, and other organic or natural impurities may be present there in drug products and other substances used in drug product use for e.g., excipients. This contamination is limited to drugs and excipient employed in the preparation of that drug.
• Separation of impurities
• Pollution can be divided into the following categories:
• Natural pollution (related to processes and drugs)
• Inanimate pollution
• Remaining solvents
Acceptance Criteria
Presence of any label-free impurities in an official item differs from the standard as if content is 0.1% or more. The total amount of all other impurities combined with the impurities detected by the monograph should not be more than 2.0%.
Tests Specific for the capsules
These tests are further categorized into two major types:
A. Physical test
B. Chemical test
Physical test
These include:
a. The Disintegration test
b. Weight Variation test
Disintegration test
It is the test that determines if the capsules are disintegrated within the prescribed period of time when they are placed in the liquid medium under the set integral conditions.
Apparatus
The equipment includes a basket-rack assembly, 1000-mL low base beaker form 139 to 160 mm high and with a diameter of 96 to 115 mm fluid immersion, thermostatic fluid setting at temperature 35 ° -39 ° and the device for enlarging and lowering basket in the subsequent immersion liquid at a frequency rate of 29 to 32 cycles/minute and distance for NLT 54mm and NMT 58 mm. The volume of liquid placed in a particular vessel is at the high point (upper blow), wire lines remain at the least 14 mm below the surface of liquid while it falls to NLT 25 mm, from the bottom of the vessel at a low blow.
Disks
Disk use is only employed when it is specified or allowed in the monograph. As specified in each monograph, each tube consists of a cylindrical disk of thickness 9.45-9.65 mm and 20.65- 20.85 mm in diameter. Each disk is made up of transparent plastic material with a specific gravity force of 1.18-1.20. Five matching holes of 1.8 to 2.1mm extend in between particular ends of the cylinder. One hole is on a circular axis, while others are like a circular axis. When disk usage is specified for each individual monograph, add a disk to each tube and use the tools as directed under each process.
The method applies to both Hard and Soft Gelatin Capsules.
Method
According to B.P, introduce each one of the capsules in the tubes, and the apparatus is suspended in the beaker carrying 60ml water at 36.5?C. in the case of hard capsules, they may float on the water surface, so the addition of a disc is mandatory. Apparatus is operated for a time period of 30min then remove apparatus assembly from the particular liquid.
Acceptance Criteria
The capsules passes test if there are No residues on the apparatus screen. On the contrary, if the residues are remaining, or it only holds fragments of the shells, i.e. the soft mass consisting of no core is obtained. If the disc is used, all the residues that are present on the lower surface consist of different fragments of the shells. In case 1/2 capsules fail the test i-e to completely disintegrate, the same test is repeated on the additional 12 capsules. NLT 16 out of 18 total capsules which are tested are completely disintegrated.
Table 4. Disintegration testing conditions and Interpretation (B.P)

The Weight Variation Test
For Hard Gelatin Capsule
Method
Usually, 20 capsules are weighed one by one so that the mean weight is determined.
Acceptance Criteria
The weight of an individual capsule should be within the limit of 90 to110% of the mean weight. If in case not all the capsules are within the specified limits, 20 capsules are weighed individually. The net or total content of each capsule is removed by using a small brush. Empty shells are weighed individually.
Net content (individual) weight= the Wt. of the shell- gross wt.
The mean total weight of the contents is calculated from the sum of the total individual weight. The particular difference between the individual and average total content is determined.
Limits
2 or not more than 2 of the mean difference is greater as compared to the 5% of the mean total content, which is not greater than 25% of the weight.
If it is more than 2, but at least not greater than 6, capsules deviate from the mean value between 10 to 25%. The total content of another 40 capsules is determined. The mean of all contents of 60 capsules is calculated. Deviation from the new average is calculated using the formula. 6 or not more out of 60 capsules should have a difference that exceeds 10% out of the average total content. Difference should never exceed more than 25%.
For the Soft Gelatin Capsule
Method
Continue as it is given under the hard capsules and calculate the total weight of the contents of each capsule. Measure the weight individually and open all capsules. Discard contents by using the particular solvents and allow all solvents to undergo the evaporation process at room temperature from shells. Shells are weighed individually. The total content is calculated.
Acceptance Criteria
It is the same as for hard gelatin capsules.
Chemical Tests
It includes the following tests:
a. Dissolution test
b. Uniformity of mass
c. Stability testing
d. Moisture Permeation test
Dissolution Test
There are 4 diff types of dissolution apparatus:
Apparatus 1: Basket apparatus
Apparatus 2: Paddle apparatus
Apparatus 3: Reciprocating cylinder
Apparatus 4: Flow-Through cell
But in capsules, the Dissolution Type 2(USP) apparatus used.
Temperature Range: 37±0.5?
Time: 4.5 hrs. If more than one time is specified, the specimen will be withdrawn at prescribed times only and within the tolerance range ±2%
Media: Acidic buffer with pH 1.3, Phosphate buffer with pH6.8. The pH should be within the range of 0.05 units as compared to the pH specified in the individual monograph.
Both the U.S.P and B.P dissolution apparatus were employed to carry out the test.
Method
The capsules are emptied in the basket, and it is immersed in the dissolution medium, rotated with a particular frequency at the specified speed in the individual monograph. The medium of dissolution apparatus is held and covered in the 1000ml glass vessel, which is maintained with a temperature 37 + 0.5?.
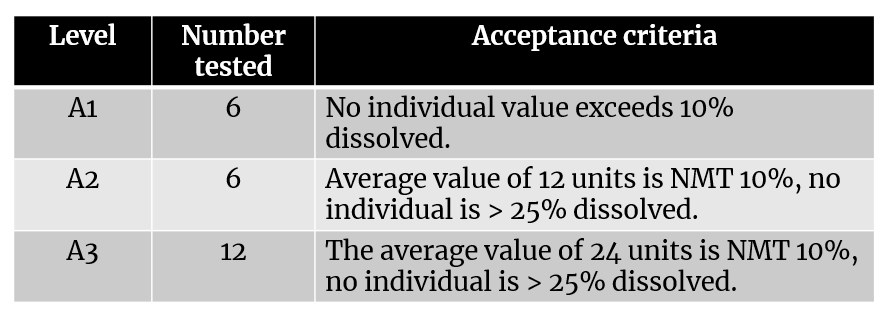
Acceptance Criteria
Acceptance criteria for immediate release hard and soft gelatin capsules.
Q = the amount of active ingredient dissolved as specified in individual monograph and is expressed as the percentage of labeled content of a particular dosage unit for e.g. 5% and 15%.
Table 5. Acceptance criteria for dissolution of capsules
|
Stage |
Number Tested |
Acceptance
Criteria |
|
S1 |
6 |
Each unit is NLT Q+5% |
|
S2 |
6 |
Average of 12 units (S1 + S2) is Equal to or
Greater than Q no Unit is Less than Q – 15% |
|
S3 |
12 |
Average of the 24 units (S1 + S2 + S3) is Equal to
or Greater than Q and not more than 2 Units is less than Q-15%, and no unit
is less than Q-25% |
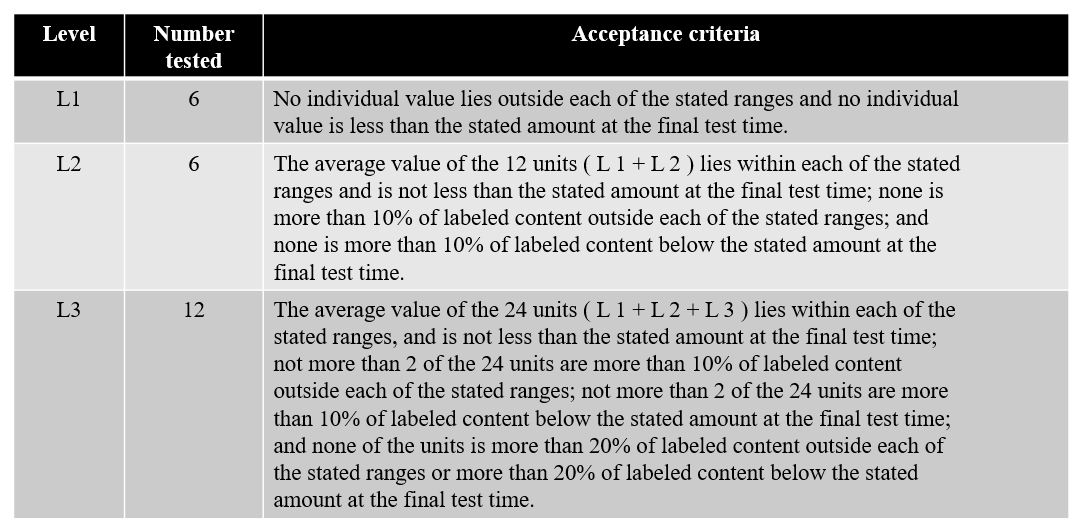
Acceptance criteria of Prolonged
Release Capsules
Acceptance
criteria of prolonged release capsules
have
limits that involve each if the Q value which is the quantity dissolved at the
specified dosing interval.
Table 6. Acceptance Criteria for the dissolution of prolonged release capsules
Dissolution test of Modified Release Capsules
There are 2 stages of dissolution test of modified release capsules.
Acid Stage
750ml/1000ml of 0.1M HCl
36.5?-37.5?
2 hrs
Using the suitable assay method analysis is performed.
Buffer Stage
Complete the operations of adding the buffer
and adjusting the pH within 5 min.
250ml of a 0.2M buffer and soln.
36.5-37.5?
6.8±0.05
45 min or for the specified time given in the individual monograph
Acceptance Criteria
For the Acid Stage
The specifications for the test are met, if they comply with the table given below. Continue testing with 3 levels unless results of both the acid and the final buffer phases correspond to the previous level
Table 7. Acceptance Criteria (acid stage) of Dissolution
For the Buffer Stage
The specifications are met if the tests comply to table below. Testing is continued through 3 levels. The Q value in the table is given i-e 75% of the dissolved, unless specified in the monograph.
Table 8. Acceptance criteria of the buffer stage of dissolution Factors affecting the dissolution in hard gelatin capsule:
Rate of dissolution is the function of:
• Shell Dissolution rate
• Penetration rate of the dissolution medium
• Disaggregation rate of the powder mass
• Primary drug particles characteristics
Uniformity of Mass
For the Buffer Stage
The specifications are met if the tests comply to table below. Testing is continued through 3 levels. The Q value in the table is given i-e 75% of the dissolved, unless specified in the monograph.
Table 8. Acceptance criteria of the buffer stage of dissolution Factors affecting the dissolution in hard gelatin capsule:
Rate of dissolution is the function of:
·
Shell
Dissolution rate
·
Penetration
rate of the dissolution medium
·
Disaggregation
rate of the powder mass
·
Primary
drug particles characteristics
Uniformity of Mass
Method
The intact capsule is weighed. The capsule is opened without the loss of any shell part, and contents are removed completely as it is possible. In the case of the soft shell capsule, firstly, wash shells with the solvent and then allow it to stand unless color of the solvent is not seen. The shell is weighed and mass of all contents is main difference between subsequent weighing. The process is repeated again by using other 19 capsules.
Acceptance Criteria
NMT 2 of individual capsule masses must deviate from mean mass by more than percentage deviations as shown in table below and none of them deviate by more than the twice of the percentage.
Table 9. Acceptance criteria for the mass uniformity of capsules
Test of Moisture Permeation
Rate or degree of the moisture permeation calculated by the
packing the units combined with a color revealing pellet desiccation. (Chang,
Raghavan, & Hussain, 1998)
Method
Expose packed unit to the known relative humidity over the
specified time. Observe desiccant pellet for the color differentiation.
Interpretation
The change in color indicates absorption of the moisture.
The pre-test weight is measured and pro-test weight of the pellet, amount is
then calculated.
|
Pharmaceutical
form |
Mean Mass |
Percentage Deviation |
|
Capsule
|
300mg or less |
10 |
|
|
300mg or above |
7.5 |
Capsule Stability Testing
Method
Gelatin capsule rapidly reach the equilibrium state with the atmospheric condition at which it is stored. The intrinsic characteristic involves a brief description of the effects of the temperature and the humidity of capsules. Given specifications as relative to the temperature and the humidity effects, on the soft type gelatin capsule are to be adjusted in order to make capsule stable, which consist of mineral oil containing the gelatin as shell and with glycerin in dry form and the (dry) gelatin in the ratio 0.6 to 1, the water- (dry) gelatin ratio 1:1 which it is dried (equilibrium) at 20 to 40% of relative humidity at temperature 20 to 44?. (Brody, Schalley, Rudkevich, & Rebek, 1999)
Interpretation
Physical stability in the case of the soft type of gelatin capsule is primarily associated with the formation and the capsule shell water losing ability. If this limitation is corrected, the upper controlled-release capsules have the satisfactory chemical stability at the S temperature from above cold to over 60. As humidity increases, moisture content of the capsules increases. For example:
Table 10. Effects on the gelatin shell with the increase of Relative Humidity
Test
Conditions
The
best results are reported by conducting at following test condition:
Table
11. Test
conditions for the stability testing of capsules
|
Relative
Humidity |
Temp. |
Effects |
|
30% |
25 |
Gelatin
retain: 12% (48mg) water Glycerin
retain: 7% (14mg) water |
|
60% |
25 |
Moisture
content: 17.4% |
|
>60% |
21-24 |
Produce good
lasting effects on shell |
|
Temp. |
Containers |
|
25 |
Open
container |
|
40 |
Open
container |
|
40 |
Closed
container (including glass bottle and a cap tight screwed) |
Results
Stability
value: 20 to 30% Relative Humidity at 21 to 24
Table
12. Temperature
and Humidity effect on the capsule shell
.

Storage of the capsules and Packing
The capsules are placed in a tightly closed container of glass and plastic, stored in a cool place. Such types of container consist of an advantage as compared to the cardboard type boxes as they are relatively lightweight and protect capsules from dust and moisture. In order to limit the shaking of capsules during transportation, the cotton piece is placed under and over the tablets or the capsules, which are mostly packed in the vials. For parts that contain highly hygroscopic types of capsules, a package containing a desiccant such as silica gel and the anhydrous form of calcium chloride can be placed in order to prevent more moisture absorption by the capsular surface. (Banker, Siepmann, & Rhodes, 2002)
Different packaging of the capsule include:
• Plastic cap screwd bottle (mostly popular packaging in the United States)
• Clam blister shell (consisting of a one-piece of plastic which folds over, then locks itself and no heating or other such process required)
• Blister packing (heat-sealed type blisters on the cardboard)
• (Plastic) Pail or the bucket (an economically best bulk form of packaging)
• Plastic locked zip pouch
Conclusion
The characterization of capsules is performed by employing universal and specific tests pripor to the batch release by the pharmaceutical industries. Medicinal and nutraceutical companies are increasingly making pharmaceutical products in the form of the capsules. This has many advantages over the tablets and it has been employed as the most commonly used adopted dosage form. An empty capsule is still available in market in a variety of different sizes, shapes most commonly each of the capsules usually contains the single dose of active ingredient. Like tablets, some encapsulated excipients can be loaded and packaged in the capsule shell. All the specification tests are performed, and acceptance criteria is quoted along with each test as mentioned in individual monograph.
References
- Banker, G. S., Siepmann, J., & Rhodes, C. (2002). Modern pharmaceutics: CRC Press.
- Brody, M. S., Schalley, C. A., Rudkevich, D. M., & Rebek, J., Julius & J Angewandte Chemie International Edition. (1999). Synthesis and characterization of a unimolecular capsule. 38(11), 1640-1644.
- Chang, R. K., Raghavan, K. S., & Hussain, M. A. J. J. o. p. s. (1998). A study on gelatin capsule brittleness: moisture tranfer between the capsule shell and its content. 87(5), 556-558.
- Chiwele, I., Jones, B. E., Podczeck, F. J. C., & Bulletin, p. (2000). The shell dissolution of various empty hard capsules. 48(7), 951-956.
- Fatohy, H. A. Course Book Industrial Pharmacy.
- Galia, E., Nicolaides, E., Hörter, D., Löbenberg, R., Reppas, C., & Dressman, J. J. P. r. (1998). Evaluation of various dissolution media for predicting in vivo performance of class I and II drugs. 15(5), 698-705.
- Hsieh, D. S. (1994). Drug permeation enhancement- theory and applications. In: Taylor & Francis.
- Samyn, J. C., & Jung, W. Y. J. J. o. p. s. (1970). In vitro dissolution from several experimental capsule formulations. 59(2), 169-175.
- Taylor, M. K., Ginsburg, J., Hickey, A. J., & Gheyas, F. J. A. P. (2000). Composite method to quantify powder flow as a screening method in early tablet or capsule formulation development. 1(3), 20-30.
- Withey, R., & Mainville, C. J. J. o. p. s. (1969). A critical analysis of a capsule dissolution test. 58(9), 1120-1126.
- Banker, G. S., Siepmann, J., & Rhodes, C. (2002). Modern pharmaceutics: CRC Press.
- Brody, M. S., Schalley, C. A., Rudkevich, D. M., & Rebek, J., Julius & J Angewandte Chemie International Edition. (1999). Synthesis and characterization of a unimolecular capsule. 38(11), 1640-1644.
- Chang, R. K., Raghavan, K. S., & Hussain, M. A. J. J. o. p. s. (1998). A study on gelatin capsule brittleness: moisture tranfer between the capsule shell and its content. 87(5), 556-558.
- Chiwele, I., Jones, B. E., Podczeck, F. J. C., & Bulletin, p. (2000). The shell dissolution of various empty hard capsules. 48(7), 951-956.
- Fatohy, H. A. Course Book Industrial Pharmacy.
- Galia, E., Nicolaides, E., Hörter, D., Löbenberg, R., Reppas, C., & Dressman, J. J. P. r. (1998). Evaluation of various dissolution media for predicting in vivo performance of class I and II drugs. 15(5), 698-705.
- Hsieh, D. S. (1994). Drug permeation enhancement- theory and applications. In: Taylor & Francis.
- Samyn, J. C., & Jung, W. Y. J. J. o. p. s. (1970). In vitro dissolution from several experimental capsule formulations. 59(2), 169-175.
- Taylor, M. K., Ginsburg, J., Hickey, A. J., & Gheyas, F. J. A. P. (2000). Composite method to quantify powder flow as a screening method in early tablet or capsule formulation development. 1(3), 20-30.
- Withey, R., & Mainville, C. J. J. o. p. s. (1969). A critical analysis of a capsule dissolution test. 58(9), 1120-1126.
Cite this article
-
APA : Pervaiz, F., Zahra, S. A., & Qaiser, F. (2018). Characterization and Evaluation of Capsules and Study of QC tests for Capsules. Global Pharmaceutical Sciences Review, III(I), 7-18. https://doi.org/10.31703/gpsr.2018(III-I).02
-
CHICAGO : Pervaiz, Fahad, Sana Ali Zahra, and Fariah Qaiser. 2018. "Characterization and Evaluation of Capsules and Study of QC tests for Capsules." Global Pharmaceutical Sciences Review, III (I): 7-18 doi: 10.31703/gpsr.2018(III-I).02
-
HARVARD : PERVAIZ, F., ZAHRA, S. A. & QAISER, F. 2018. Characterization and Evaluation of Capsules and Study of QC tests for Capsules. Global Pharmaceutical Sciences Review, III, 7-18.
-
MHRA : Pervaiz, Fahad, Sana Ali Zahra, and Fariah Qaiser. 2018. "Characterization and Evaluation of Capsules and Study of QC tests for Capsules." Global Pharmaceutical Sciences Review, III: 7-18
-
MLA : Pervaiz, Fahad, Sana Ali Zahra, and Fariah Qaiser. "Characterization and Evaluation of Capsules and Study of QC tests for Capsules." Global Pharmaceutical Sciences Review, III.I (2018): 7-18 Print.
-
OXFORD : Pervaiz, Fahad, Zahra, Sana Ali, and Qaiser, Fariah (2018), "Characterization and Evaluation of Capsules and Study of QC tests for Capsules", Global Pharmaceutical Sciences Review, III (I), 7-18
-
TURABIAN : Pervaiz, Fahad, Sana Ali Zahra, and Fariah Qaiser. "Characterization and Evaluation of Capsules and Study of QC tests for Capsules." Global Pharmaceutical Sciences Review III, no. I (2018): 7-18. https://doi.org/10.31703/gpsr.2018(III-I).02
